Chapter 6 notes
advertisement

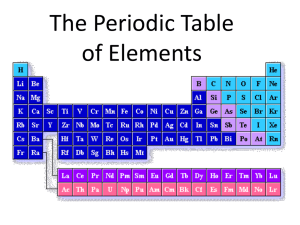
Chapter 6 The Periodic Table Section 1 Development of the Modern Periodic Table Section 1: Development of the Modern Periodic Table The periodic table evolved over time as scientists discovered more useful ways to compare and organize the elements. K What I Know W What I Want to Find Out L What I Learned • In 1750, only _____ elements were known. • As the rate of discovery increased, so did the need to organize the elements • In 1789 Antoine Lavoisier grouped the known elements into metals, nonmetals, gases, and earths. Development of the Periodic Table • In the 1700s, Lavoisier compiled a list of all the known elements of the time. Development of the Modern Periodic Table Development of the Periodic Table • The 1800s brought large amounts of information and scientists needed a way to organize knowledge about elements. • ____________proposed an arrangement where elements were ordered by increasing atomic mass. Development of the Modern Periodic Table Development of the Periodic Table • Newlands noticed when the elements were arranged by increasing atomic mass, their properties repeated every eighth element. Development of the Modern Periodic Table Development of the Periodic Table • Meyer and Mendeleev both demonstrated a connection between atomic mass and elemental properties. • Moseley rearranged the table by increasing atomic number, and resulted in a clear periodic pattern. • Periodic repetition of chemical and physical properties of the elements when they are arranged by increasing atomic number is called _____________. Development of the Modern Periodic Table Mendeleev’s Periodic Table • ___________made flash cards of the 63 known elements. (1863) – On each card he put the name of the element, mass, and properties. – When he lined the cards up in order of increasing mass, a pattern emerged. – Mendeleev arranged the elements into row in order of increasing mass so that elements with similar properties were in the same column. A deck of cards can be divided into four suits—diamonds, spades, hearts, and clubs. In one version of solitaire, a player must produce an arrangement in which each suit is ordered from ace to king. This arrangement is a model for Mendeleev's periodic table. • _______________- Arrangement of elements in columns, based on a set of properties that repeat from row to row. • Mendeleev’s Prediction – He could not make a complete table because many of the elements had not yet been discovered. He had to leave spaces for those elements. • Eka-Aluminum – one space below Al. He predicted it would be a soft metal with a low m.p. and a density of 5.9 g/cm3 – The close match between Mendeleev’s prediction and the actual properties of new elements showed how useful the periodic table could be. • Gallium was discovered in 1875. It’s a soft metal, m.p. is 29.7 ˚C, and has a density of 5.91 g/cm3 Heat from a person's hand can melt gallium. In some traffic signals, there are tiny light emitting diodes (LEDs) that contain a compound of gallium Mendeleev’s Periodic Table • How is the table organized? – Elements are arranged in order of increasing ___________ • What do the long dashes represent? – They represent _______________elements. • Why are masses listed with some of the dashes, but not with all of them? – He was able to predict properties for some unknown elements based on the properties of neighboring elements. Development of the Modern Periodic Table The Modern Periodic Table • The modern periodic table contains boxes that contain the element's name, symbol, atomic number, and atomic mass. Development of the Modern Periodic Table The Modern Periodic Table • Columns of elements are called _____________. • Rows of elements are called _________________. • Elements in groups 1,2, and 13–18 possess a wide variety of chemical and physical properties and are called the ____________________elements. • Elements in groups 3–12 are known as the __________________metals. Development of the Modern Periodic Table The Modern Periodic Table • Elements are classified as metals, nonmetals, and metalloids. • _______are elements that are generally shiny when smooth and clean, solid at room temperature, and good conductors of heat and electricity. • _____metals are all the elements in group 1 except hydrogen, and are very reactive. • ___________earth metals are in group 2, and are also highly reactive. Development of the Modern Periodic Table The Modern Periodic Table • The transition elements are divided into __________metals and inner transition metals. • The two sets of inner transition metals are called the ________series and ___________series and are located at the bottom of the periodic table. Development of the Modern Periodic Table The Modern Periodic Table • ____________are elements that are generally gases or brittle, dull-looking solids, and poor conductors of heat and electricity. • Group 17 is composed of highly reactive elements called ___________. • Group 18 gases are extremely unreactive and commonly called __________gases. • ___________, such as silicon and germanium, have physical and chemical properties of both metals and nonmetals. Development of the Modern Periodic Table Staircase • Left side is metals: Elements to the left of the semimetal line on the periodic table are malleable (can be hammered into a shape), ductile (can be stretched into a wire) and good conductors of heat and electricity. These elements tend to lose electrons to form cations. • Right side is nonmetals: Elements to the right of the semi-metal line on the periodic table (and hydrogen) are brittle and insulators of heat and electricity. These elements tend to gain electrons to form anions or share electrons as bonds to form molecules. • On the steps is metaloids The Modern Periodic Table Development of the Modern Periodic Table Essential Questions • How was the periodic table developed? • What are the key features of the periodic table? Development of the Modern Periodic Table Section 2 Classification of the Elements Section 2: Classification of the Elements Elements are organized into different blocks in the periodic table according to their electron configurations. K What I Know W What I Want to Find Out L What I Learned Organizing the Elements by Electron Configuration • Recall electrons in the highest principal energy level are called _______electrons. All group 1 elements have one valence electron. Classification of the Elements Organizing the Elements by Electron Configuration • Group 2 elements have ____valence electrons. The number of valence electrons for elements in groups 13–18 is ten less than their group number. The energy level of an element’s valence electrons indicates the period on the periodic table in which it is found. Classification of the Elements The s-, p-, d-, and f-Block Elements • The shape of the periodic table becomes clear if it is divided into blocks representing the atom’s ________sublevel being filled with valence electrons. Classification of the Elements The s-, p-, d-, and f-Block Elements • s-block elements consist of group ___and ____, and the element helium. • Group 1 elements have a partially filled _____orbital with one electron. • Group 2 elements have a completely filled _____orbital with two electrons. Classification of the Elements The s-, p-, d-, and f-Block Elements • Groups 13–18 fill the ___ orbitals. In group 18, both the s and p orbitals of the period’s principal energy level are completely filled. Classification of the Elements The s-, p-, d-, and f-Block Elements • The d-block contains the __________metals and is the largest block. • There are exceptions, but d-block elements usually have filled outermost s orbitals, and filled or partially filled d orbitals. • The five d orbitals can hold 10 electrons, so the d-block spans ten groups on the periodic table. Classification of the Elements The s-, p-, d-, and f-Block Elements • The f-block contains the inner transition metals. • f-block elements have filled or partially filled outermost s orbitals and filled or partially filled 4f and 5f orbitals. • The 7f orbitals hold 14 electrons, and the inner transition metals span 14 groups. Classification of the Elements Alkali Metals Element Symbol Hyperlink Lithium Li Sodium Na • _____Valence Electron • Found in nature only in a compound. • Form +1 ions because they will easily give up 1 electron for stability. Potassium K Rubidium Rb Cesium Cs • http://www.youtube.com/watch?v=Ft4E1eCUItI • http://www.youtube.com/watch?v=eCk0lYB_8c0 Francium Fr • Group 1A • Most _______metals – Reactivity increases from the top to the bottom. – So reactive many are kept under oil to prevent reacting with water or oxygen. Alkaline Earth Metals • Group 2A • Have ____Valence Electrons • Harder than the metals in 1A. • Form ___ Ions because they easily give up 2 electrons for stability. • Magnesium used in photosynthesis within the chlorophyll. • Calcium used in teeth and bone. • Element Symbol Hyperlink Beryllium Be Magnesium Mg Calcium Ca Strontium Sr Barium Ba Radium Ra http://www.youtube.com/watch?v=B2ZPrg9IVEo Boron Family Group 3A Have __ Valence electrons Form __ Ions because they easily give up 3 electrons for stability. 1 metalloid (Boron) Six metals Aluminum is the most abundant metal in the Earth’s crust. People are encouraged to recycle aluminum because it doesn’t take that much energy to do so. Element Symbol Hyperlink Boron B Aluminum Al Gallium Ga Indium In Thallium Tl Ununtrium Uut Carbon Family Group 4A Have __ Valence Electrons Form _____ Ions because it will easily lose or gain 4 electrons for stability. 1 Nonmetal (Carbon) 2 Metalloids 3 Metals Metallic nature increases from top to bottom. With the exception of water, most of the compounds in your body contain carbon. Silicon is the second most abundant metal in the earth’s crust. Element Symbol Hyperlink Carbon C Silicon Si Germanium Ge Tin Sn Lead Pb Ununquadium Uuq Nitrogen Family • Group 5A Element • Have ___ Valence Electrons • Forms __ Ions because Nitrogen it will easily gain 3 Phosphorus electrons for stability. • 2 nonmetals Arsenic • 2 metalloids Antimony • 2 Metals Bismuth • Nitrogen and Phosphorus are used Ununpentium in fertilizers. Symbol Hyperlink N P As Sb Bi Uup Oxygen Family Group 6A Have __ Valence Electrons Forms ___ Ions because it will easily gain 2 electrons for stability. 3 nonmetals 2 metalloids 1 metal Oxygen is the most abundant element in the Earth’s Crust. Ozone is another from of oxygen. At ground level it can irritate your eyes and lungs. At higher levels it absorbs harmful radiation from the sun. Element Symbol Hyperlink Oxygen O Sulfur S Selenium Se Tellurium Te Polonium Po Ununhexium Uuh Halogens Group 7A Have ___ Valence electrons Form ___ Ions because it will easily gain 1 electron for stability. Most reactive nonmetals increase from bottom to top. Known as “Salt Formers” 5 nonmetals 1 Unknown Fluorine is the most reactive. React easily with most metals. http://www.youtube.com/watch?v=u2ogMUDBaf4 http://www.youtube.com/watch?v=yP0U5rGWqdg • Element Symbol Hyperlink Fluorine F Chlorine Cl Bromine Br Iodine I Astatine At Ununspetium Uus Noble Gases • Group 8A • ___ Valence Electrons • _______is the exception with only 2 valence electrons. • Extremely Un-reactive (Do not form Ions) • Odorless and colorless. • Used in light bulbs. • All are used in neon lights except argon. • Have the most stable electron configuration. • http://www.youtube.com/watch?v=QLrofyj6a 2s Element Helium Symbol Hyperlin k He Neon Ne Argon Ar Krypton Kr Xenon Xe Radon Rn Ununoctium Uuo Electron Configuration and the Periodic Table Use with Example Problem 1. Problem Strontium, which is used to produce red fireworks, has an electron configuration of [Kr]5s2. Without using the periodic table, determine the group, period, and block of strontium. Classification of the Elements Essential Questions • Why do elements in the same group have similar properties? • Based on their electron configurations, what are the four blocks of the periodic table? Classification of the Elements Section 3 Periodic Trends Section 3: Periodic Trends Trends among elements in the periodic table include their sizes and their abilities to lose or attract electrons. K What I Know W What I Want to Find Out L What I Learned Atomic Radius • Atomic size is a periodic trend influenced by electron configuration. • For metals, atomic radius is half the _______between adjacent nuclei in a crystal of the element. Periodic Trends Atomic Radius • For elements that occur as molecules, the atomic radius is half the distance between nuclei of identical atoms that are chemically bonded together. Periodic Trends Atomic Radius • Atomic radius generally _________from left to right, caused by increasing positive charge in the nucleus. • Valence electrons are not shielded from the increasing nuclear charge because no additional electrons come between the nucleus and the valence electrons. • Atomic radius generally _________as you move down a group. • The outermost orbital size increases down a group, making the atom larger. Periodic Trends Atomic Radius Periodic Trends Interpret Trends in Atomic Radii Use with Example Problem 2. Problem Which has the largest atomic radius: carbon (C), fluorine (F), beryllium (Be), or lithium (Li Explain your answer in terms of trends in atomic radii. Periodic Trends Ionic Radius • An _____is an atom or bonded group of atoms with a positive or negative charge. • When atoms lose electrons and form positively charged ions, they always become smaller for two reasons: 1. The loss of a valence electron can leave an empty outer orbital, resulting in a ___________radius. 2. Electrostatic repulsion decreases allowing the electrons to be pulled closer to the nucleus. Periodic Trends Ionic Radius • When atoms gain electrons, they can become ____, because the addition of an electron ____________electrostatic repulsion. Periodic Trends Ionic Radius • The ionic radii of positive ions generally ________from left to right. • The ionic radii of negative ions generally ________from left to right, beginning with group 15 or 16. • Both positive and negative ions __________in size moving down a group. Periodic Trends Ionic Radius Periodic Trends Ionization Energy • ________________is defined as the energy required to remove an electron from a gaseous atom. • The energy required to remove the first electron is called the first _________ energy. Periodic Trends Ionization Energy Periodic Trends Ionization Energy • Removing the second electron requires more energy, and is called the second ionization energy. • Each successive ionization requires more energy, but it is not a steady increase. Periodic Trends Ionization Energy • First ionization energy _________from left to right across a period. • First ionization energy ___________down a group because atomic size increases and less energy is required to remove an electron farther from the nucleus. Periodic Trends Ionization Energy • The ionization at which the large increase in energy occurs is related to the number of valence electrons. • The ________states that atoms tend to gain, lose or share electrons in order to acquire a full set of eight valence electrons. • The _________is useful for predicting what types of ions an element is likely to form. Periodic Trends Electronegativity • The ________________of an element indicates its relative ability to attract electrons in a chemical bond. • Electronegativity ________down a group and increases left to right across a period. Periodic Trends Patterns on the Periodic Table 1. 2. 3. 4. 5. 6. 7. 8. 9. Atomic # L to R. Atomic mass L to R. Energy level and orbitals in rows from T to B. (Physical Properties) metals metalloids nonmetals from L to R. Columns atomic mass from T to B. Columns are based on chemical properties (reactivity). Valence Electrons from L to R. Most reactive metals are on the left side. Most reactive non-metals are on the right side. Essential Questions • What are the period and group trends of different properties? • How are period and group trends in atomic radii related to electron configuration? Periodic Trends