5-_Diagnostic_Microbiology
advertisement

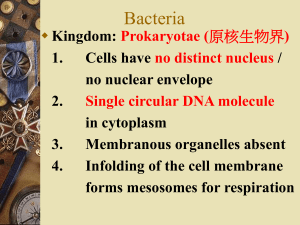
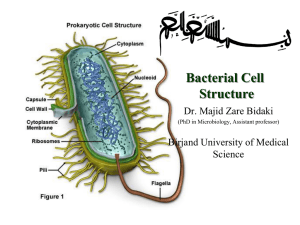
Bacteria are either identified in A pathological specimen obtained from the patient (e.g. pus, sputum, urine, blood, stools, etc.) depending on the site of infection After been grown on artificial nutrient media Bacteria are then identified by Microscopic Examination Examination of fresh samples used for demonstration of bacterial motility using hanging drop method Morphology and staining reactions of bacteria The hanging Drop Method Commonly used stains 1- Simple stains e.g. methylene blue 2- Differential stains e.g. Gram stain Primary stain Methyl violet (Crystal Violet)- Iodine mixture Decolourization Alcohol Counter stain Diluted carbol-fuchsin stain (Safranin) Results Gram (+) Gram (-) Purple Red Difference due to structure of cell wall Gram (+) Gram (-) Thick cell wall Thin cell wall A Gram stain of mixed Staphylococcus aureus Gram’s Stain Ziehl–Neelsen stain Differential Stain - divides bacteria into 2 groups Acid Fast Non Acid Fast Used to identify organisms in the Genera Mycobacterium (high lipid and wax content in cell wall) Procedure Fix the smear of the specimen over the glass slide either by heating or alcohol fixation Pour carbol fuschin over smear heat gently until fumes appear do not overheat allow it to stand for 5 minutes wash it off with water Pour 20% sulphuric acid 5% sulfuric acid is used for destaining Mycobacterium leprae instead of the 20% used for Mycobacterium tuberculosis wait for one minute keep on repeating this step until the slide appears light pink in color wash off with water Pour methylene blue wait for two minutes again wash with water Allow it to air dry examine under oil immersion lens Result Acid Fast organism Red as Mycobacterium tuberculosis Non Acid Fast organism Blue as Enterobacteriaceae family A. Non Acid-fast bacteria B. Acid-fast bacteria Mycobacterium tuberculosis (stained red) in tissue (blue) Special stains Capsule stain and Flagella stain Pseudomonas fluorescens cultured on nutrient agar, stained using the Presque Isle flagella stain Encapsulated Bacillus sp. stained using Maneval's capsule staining method (II) Cultural Characters Bacteria need nutritive culture media to multiply in vitro An undefined medium (also known as a basal or complex medium). It is a medium that contains: 1- A carbon source such as glucose for bacterial growth 2- Water 3- Various salts needed for bacterial growth Defined media (also known as chemically defined media or synthetic media) Classification of Media Media can be classified into 1-Minimal media ( simple medium) It contains the basic nutritive requirements e.g. nutrient broths and agar media 2- Selective media Selective media are used for the growth of only selective microbes It contains antibiotics, dye, or specific chemicals inhibits the growth of most types of microbe stimulate the isolation of one type Mannitol salt agar (MSA) selective for Gram positive (+ve) bacteria An MSA plate with Micrococcus sp. (1), Staphylococcus epidermis (2) and S. aureus colonies (3). Blood-free, charcoal-based selective medium agar (CSM) isolation of Campylobacter sp. Blood-free, charcoal-based selective medium agar (CSM) for isolation of Campylobacter. Löwenstein–Jensen medium enriched selective media for T.B. Distinctive clusters of colorless Mycobacterium tuberculosis Löwenstein-Jensen medium used for growing M. tuberculosis in a McCartney bottle TCBS agar (Thiosulfate-citrate-bile salts-sucrose agar) selective for Vibrio cholerae due to alkaline pH Yellow coloured (sucrose fermenting) colonies of Vibrio cholerae on TCBS agar. 3-Differential media Differential media or indicator media distinguish one microorganism type from another growing on the same media Indicators neutral red phenol red eosin Y methylene blue Examples of differential media include Eosin methylene blue (EMB) differential for lactose and sucrose fermentation E. coli on EMB agar MacConkey (MCK) differential for lactose fermentation A MacConkey agar plate with an active bacterial culture 4- Enriched media Enriched media contain the nutrients required to support the growth of a wide variety of organisms including some of the more fastidious ones Blood agar Is an enriched medium in which nutritionally rich whole blood supplements the basic nutrients It contains 5-10% human or animal blood It shows the type of haemolytic activity of bacteria (complete, partial or non-haemolytic) Complete Haemolysis of RBCs (Beta Haemolytic Streptococci) Partial Haemolysis of RBCs (Alpha Haemolytic Streptococci) Chocolate agar (heated blood agar) enriched with heat-treated blood (40-45°C). Comparison of two culture media types used to grow Neisseria gonorrhoeae bacteria Lofflers serum media Horse serum + glucose in a ratio 3:1 It is used for cultivation of Corynebacterium diphtheriae 5- Transport media Transport medium is a simple organic medium maintain the viability of all organisms in the specimen without altering their concentration This type of medium mainly used for temporary storage of specimens being transported to the laboratory for cultivation Examples of transport media include Thioglycollate broth for strict anaerobes Thioglycollate broth medium is recommended to isolate strict anaerobes should an anaerobic infection be suspected The colonial appearance on culture media Shape The colonies may be small (pin-point) fimbriate, flat or convex Colour The colonies may be colorless or bacteria produce endopigments which give the colonies a characterestic colour Staph. aureus produce golden yellow colonies Staph. albus produce white endopigment Staph. citreus produce a lemon yellow endopigment The bacteria may produce exopigments Pseudomonas aeruginosa produce a green exopigments in the surrounding media Antimicrobial Chemotherapy An antibacterial agent is a compound or substance that kills or slows down the growth of bacteria Antibiotic(s) has come to include a broader range of antimicrobial compounds, including anti-fungal and other compounds It is produced by microbes and is harmful to other microbes, except viruses These include beta-lactam antibacterial penicillin (produced by Penicillium notatum) cephalosporin Compounds that are still isolated from living organisms Aminoglycosides Other chemotherapeutic agents produced by chemical synthesis Sulfonamides Quinolones Classification of Antibiotics According to agent action Antibacterial agents are divided into two broad groups based on their biological effect on microorganisms bactericidal agents kill bacteria bacteriostatic agents slow down or stall bacterial growth Bactericidal antibiotics Antibiotics that inhibit cell wall synthesis Beta-lactam antibiotics penicillin derivatives, and cephalosporins Aminoglycosidic antibiotics are usually considered bactericidal although they may be bacteriostatic with some organisms Bacteriostatic antibiotics limit the growth of bacteria by interfering with bacterial protein production DNA replication Or other aspects of bacterial cellular metabolism This group includes Tetracyclines Sulphonamides Trimethoprim Chloramphenicol Macrolides Antibiotic sensitivity test Antibiotic sensitivity is a term used to describe the susceptibility of bacteria to antibiotics Antibiotic susceptibility testing (AST) is usually carried out to determine which antibiotic will be most successful in treating a bacterial infection in vivo Testing for antibiotic sensitivity is often done by the Kirby-Bauer method ( Disc-diffusion method) Other methods to test antimicrobial susceptibility include the E-test (also based on antibiotic diffusion) Agar and Broth dilution methods for Minimum Inhibitory Concentration determination In Kirby-Bauer testing, white wafers containing antibiotics are placed on a plate of bacteria. Circles of poor bacterial growth surround some wafers indicating susceptibility to the antibiotic. This is most commonly used in the setting of medicine, where a particular organism has been found to infect a patient, and the doctor treating the patient is seeking guidance on what concentration of antibiotic is suitable. The Dilution Method Serial dilutions of antibiotics are incorporated in agar containing or broth culture media The lowest concentration of antibiotic that prevents visible growth after an 18-24 hours incubation period is known as minimal inhibitory concentration (MIC) The minimal bactericidal concentration (MBC) may be determined in broth dilution tests by subculturing the containers that show no growth on to antibiotic-free agar containing media The lowest concentration of antibiotic that totally suppresses growth after overnight incubation is known as MBC Minimum Inhibitory Concentration