Lipids
Chapter 29
Lipids
• Lipids are biomolecules that are soluble in organic solvents.
• The identity of lipids is defined on the basis of a physical property and
not by the presence of a particular functional group.
• Lipids share many properties with hydrocarbons.
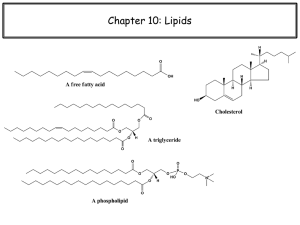
Figure 29.1
Three examples of lipids
2
Hydrolyzability of Lipids
• Lipids can be categorized as hydrolyzable and nonhydrolyzable.
[1] Hydrolyzable lipids can be cleaved into smaller molecules by hydrolysis
with water.
• Many hydrolyzable lipids contain an ester unit.
[2] Nonhydrolyzable lipids cannot be cleaved into smaller units by
aqueous hydrolysis.
3
Waxes
Mostly found as mixtures
• Esters of High Mw alcohol and High Mw acid
• Crystalline pure, plastic and malleable as mixtures
Spermacetti (whale oil) – buoyancy mechanism for diving (mix freezes at 30 °C)
Bees Wax (mp 60-64 °C), Carnauba (Brazil or palm) wax
Also jojoba wax (C36-C46 acids & alcohols)
Sebum (includes 20-30% waxes)
Cerotic acid C26 carboxylic acid
Essential
Fatty acids
(no
biosynthesis
in humans)
Triglycerides (oils and fats)
•Fats melt above room temperature
•Oils melt below room temperature
•Unsaturated is always (Z) or (cis)
•Unsaturated have lower melting points
than saturated
(butter is a solid, olive oil is a liquid)
•More unsaturation the lower the melting
point.
Triglycerides: Cocoa butter
Oleic, palmitic, steric esters
Mixture of triglycerides
Melts 34 °C (in your mouth)
Fish oils (cod liver and herring oils) unsaturated to remain liquid in cold water
Hydrolysis of Triglycerides
Mechanism for hydrolyses?
Reactions of unsaturated fatty esters
• Hydrolysis, transesterification
• Reduction of C=C
• Allylic Oxidations
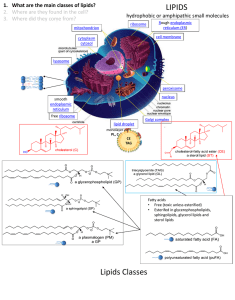
Phospholipids (hydrolyzable)
phosphoacylglycerols
phosphatidylethanolamine
or cephalin
phosphatidylcholine
or lecithin
sphingomyelins
myelin sheath around nerves is rich
in sphingomyelins
derivatives of the amino alcohol sphingosine
3D Structure of Phosphoacylglycerols
Fat soluble Vitamins: A & D
• organic compounds required in small quantities for normal
metabolism. Not synthesized by organism. Must come from diet.
Deficiency: Night blindness
intestinal absorption of calcium
and phosphate
Deficiency: Rickets
Fat soluble Vitamins: E & K
Antioxidant
Deficiency: neurological problems
Blood clotting
Deficiency: excessive bleeding
Eicosanoids
• group of potent biologically active compounds containing 20
carbon atoms derived
from arachidonic acid.
• prostaglandins, leukotrienes, thromboxanes, and prostacyclins
• Autocrine or paracrine hormones
Lower blood pressure
Inhibit platelet aggregation
Control inflammation
Lower gastric secretions
Induce labor
Control cell growth
Control calcium transport
Sensitizes spinal neurons to pain
Constrict smooth muscle (lungs)
Dilate blood vessels
Inhibit platelet aggregation
Constrict blood vessels
Trigger platelet aggregation
Prostaglandin Analogs
• Prostaglandins themselves are unstable in the body, having a half-life of
only minutes.
• Thus, more stable analogues have been developed that retain their
biological activity for longer periods.
• An example is misoprostol, an analogue of PGE1, which is sold as a
mixture of stereoisomers.
• Misoprostol is administered to prevent gastric ulcers in patients who are
at high risk of developing them.
16
Synthesis of Eicosanoids
Figure 29.7
The conversion of arachidonic
acid to prostaglandins,
thromboxanes, prostacyclins,
and leukotrienes
•Aspirin and other nonsteroidal antiinflammatory drugs
(NSAID) inactivate COX
1 & 2 enzymes
• results in an increase
in gastric secretions
17
COX-2 Inhibitors
• A group of anti-inflammatory drugs that block only the COX-2 enzyme
were developed in the 1990’s.
• These drugs—rofecoxib, valdecoxib, and celecoxib—do not cause an
increase in gastric secretions, and were thus believed to be especially
effective for long-term use in patients with arthritis.
• Unfortunately, both rofecoxib and valdecoxib have been removed from
the market, since their use has been associated with increased risk of
heart attack and stroke.
18
Terpenes
• Terpenes are lipids composed of repeating five-carbon units called
isoprene units.
• An isoprene unit has five carbons: four in a row, with a one-carbon
branch on a middle carbon.
• Terpenes can be cyclic or acyclic, and may contain heteroatoms.
19
Indentifying terpenes
Squalene in sebaceous oils
Terpene biosynthesis (carbocation
chemistry)
Terpene biosynthesis
All other terpenes are formed from farnesyl and geranyl diphosphates
24
Formation of cyclic terpenes
Steroids
Hormones
Surfactants (membranes)
Oils
Cholesterol
8 chiral centers
28 =256 possible
stereoisomers
biosynthesis in the body from squalene (C30).
Biosynthesis of Cholesterol
More stable
Cholesterol-Lowering Drugs
• Several drugs are now available to reduce the level of cholesterol in the
bloodstream.
• These compounds act by blocking the biosynthesis of cholesterol in its
early stages.
• Two examples are atorvastatin (Lipitor) and simvastatin (Zocor).
Figure 29.12
29
Steroidal Sex Hormones
30
Synthetic Hormone Steroids
• Synthetic analogues of these steroids have found important uses, such
as in oral contraceptives.
• Synthetic androgen analogues, called anabolic steroids, promote muscle
growth.
• Although they are used by athletes, their use is not permitted in
competitive sports.
31
Adrenal Cortical Steroids
• A second group of steroid hormones includes the adrenal cortical
steroids.
• Examples are cortisone, cortisol, and aldosterone.
• All of these compounds are synthesized in the outer layer of the adrenal
gland.
• Cortisone and cortisol serve as anti-inflammatory agents and they also
regulate carbohydrate metabolism.
• Aldosterone regulates blood pressure and volume by controlling the
concentration of Na+ and K+ in body fluids.
32
Lanolin
“lana” wool
“Olin” from oleum for oil
5-25 wt% of wool is lanolin oil
R = C10-C22
40% alpha hydroxyesters
No triglycerides
Waxes
Cholesterol esters
Sebaceous glands