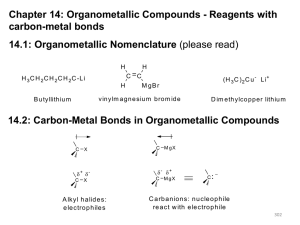
Chapter 17 Carbonyl Compounds II 17.2
advertisement

Main Menu 17.2 How Aldehydes and Ketones React (Part I) d- Electron rich (Lewis base, Nu) d+ Electron deficient (Lewis acid, E+) R = alkyl or aryl (C) Y = alkyl, aryl or H (class II) (No leaving group) 1 Class I vs. Class II Carbonyl Compounds Class I Y = NR’2 (amide) = OR’ (ester, carboxylic acid) = OCOR’ (acid anhydride) = X (acyl halide) Class II Y = H (aldehyde) = R’’ (ketone) H-H (pKa = 35) R-H (pKa = 50) Hydride (H-) and carboanion are not leaving groups 2 Relative Reactivity of Class I and Class II Carbonyl Compounds > acyl halide >> acid anhydride > H aldehyde > R’ ketone > ester amide Esters and amides are more stable than ketones and aldehydes due to their resonance stabilization. 3 Nucleophilic Addition (Class II) 1. General mechanism in basic condition: 2. General mechanism in acidic condition: 4 Important pKa to Remember Acids H-Z Names Alkane (2°) H 3C CH H H 3C H Amine N H H Hydrogen 51 38 H Carboxylic Acid Hydrochloric Acid H 3C CH H 3C H Base as Li+ salt Nucleophile as Grignard reagent Base in NaH, CaH2 Nucleophile in LiAlH4, NaBH4 H Often as a base but can be a nucleophile 15-16 R NH R General Roles of :Z Base and Nucleophile N 35 H 10-11 H H Thiol Conjugate Base, :Z H Alcohol water Ammonium Approx. pKa S RCO Cl 2 R NH H H 10-11 H 4-5 RCO -7 Cl H R Weak base, but can be a nucleophile Nucleophile S 2 Weak base, poor leaving group Leaving group, poor nucleophile 5 Types of Nucleophile for Class II Carbonyl Groups 1. Carbon as the nucleophilic atom Basic condition pKa = 25 pKa = 50 carboanion Acetylide ion 2. Hydrogen as the nucleophilic atom hydride Mostly basic condition 3. Nitrogen as the nucleophilic atom 1° and 2° amines Mostly acidic condition 4. Oxygen as the nucleophilic atom 1° alcohols Acidic condition 6 Carbon as the Nucleophilic Atom: Grignard Reagents Carboanions are highly reactive. Hard to find a base to do the deprotonation. pKa = 50 carboanion Formation of Grignard reagent THF or Et2O X = Cl, Br or I The carbonanions can be stabilized. THF: tetrahydrofuran Et2O: diethyl ether 7 Reactions of Grignard Reagents 8 Reactions of Grignard Reagents 3° alcohols 2° alcohols 9 Reactions of Grignard Reagents 1° alcohols (one extra carbon) 1° alcohols (two extra carbons) Carboxylic acid 10 Reactions of Grignard Reagents with Esters 1 mol. 0.5 mol. 1 mol. 0.5 mol. 1 mol. 2 mol. 1 mol. 11 Reactions of Grignard Reagents with Esters Why two equivalents of Grignard reagent are needed? A ketone (more reactive than ester) 12 Carbon as the Nucleophilic Atom: Acetylide Ions pKa = 25 pKa = 50 carboanion Acetylide ion Why the pKa of acetylide is much lower? 2Pz 2S 2Py The radius of 2S orbital is smaller than the radius of 2P orbitals. 2Px Order for the radius of hybridized orbitals: SP3 > SP2 > SP Order for the electronegativity of hybridized orbitals: SP3 < SP2 < SP Order for the acidity of H’s of hybridized orbitals: SP3 < SP2 < SP pKa = 40 13 Reactions of Carbonyl Groups with Acetylide Ions pKa = 25 Acetylide ion pKa = 38 14 Carbon as the Nucleophilic Atom: Cyanide Hydrogen cyanide is weakly acidic. pKa = 9.1 cyanide Cyanide is highly poisonous. Addition of cyanide to aldehydes or ketones: HCl H+, H2O heat a-hydroxy carboxylic acid Stable in acidic condition but unstable in basic condition. H2, Pt/C 15 Hydrogen as the Nucleophilic Atom: Hydride Reagents Reagents that can provide hydrides as nucleophiles: NaBH4 LiAlH4 Sodium boroydride Lithium aluminum hydride Theoretically, one molecule of LiAlH4 or NaBH4 can provide four hydrides. Diisobutylaluminum hydride (DIBAL) Reagents that can provide hydrides as bases: NaH CaH2 16 Reactions of Aldehydes and Ketones with Hydride Reagents General Reactions: 1) LiAlH4 or NaBH4 2) H2O Examples: 17 General Mechanism for the Reduction of Aldehydes and Ketones Using Hydride Reagents d- Repeat 3 times d- The three H’s can still act as hydrides. H2O 18 Comparison of LiAlH4, DIBAL and NaBH4 Relative Reactivity LiAlH4 > DIBAL > NaBH > NaBH CN 4 Unstable in weak acid 3 Stable in weak acid Amide Ester Carboxylic acid Ketone Aldehyde LiAlH4 yes yes yes yes yes DIBAL no yes ? yes yes NaBH4 no no no yes yes 19 Reduction of Ester with LiAlH4 General reaction 1) LiAlH4 2) H2O Mechanism Reduction cannot stop at the stage of aldehyde H2O 20 Reduction of Carboxylic Acids with LiAlH4 General reaction 1) LiAlH4 2) H2O Reduction cannot stop at the stage of aldehyde Mechanism H2O 21 Reduction of Amides with LiAlH4 General reaction 1) LiAlH4 2) H2O Mechanism H2O 22 Reduction of Ester with DIBAL General reaction 1) DIBAL, -78°C 2) H2O, -78°C Reduction can stop at the stage of aldehyde 1) DIBAL, -78° - 0°C 2) H2O, 0°C Control of temperature is important for the reduction to stop at the stage of aldehyde. 23 Examples 24 Examples 25 Examples No reaction No reaction 26 Selective Reduction In most of the cases, hydride reducing reagents cannot reduce C=C. 27 Learning Check 1. What could be the reagent needed for this transformation? 2. What could be reagent needed for this transformation? 28 Learning Check 3. What could be the product for the following reaction? 4. What could be the product for the following reaction? 29 Learning Check 5. What could be the reagent needed for the following reaction? 6. What could be the product for the following reaction? 30 Learning Check 7. What could be the product for the following reaction? O OCH3 (a) OH 1) CH3CH2MgBr (2 equivalents) 2) HCl, H2O (b) product ? O (c) OH O (d) CH2CH3 OH CH2CH3 (e) none of the above 31 Learning Check Main Menu 8. What could be the product for the following reaction? 32