Ch. 12
advertisement

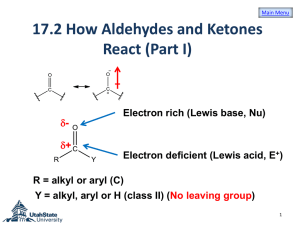
Chapter 12 Alcohols from Carbonyl Compounds Oxidation-Reduction & Organometallic Compounds 1. Structure of the Carbonyl Group Carbonyl compounds O R O H R Aldehyde R' Ketone O R O O OH Carboxylic acid R O OR' Ester R N R' Amide R" Ch. 12 - 2 Structure ~ 120o O C ~ 120o ~ 120o ● Carbonyl carbon: sp2 hybridized ● Planar structure Ch. 12 - 3 Polarization and resonance structure O C O C Ch. 12 - 4 1A. Reactions of Carbonyl Compounds with Nucleophiles One of the most important reactions of carbonyl compounds is nucleophilic addition to the carbonyl group O Nu C O nucleophilic addition Nu C Ch. 12 - 5 Two important nucleophiles: ● Hydride ions (from NaBH4 and LiAlH4) ● Carbanions (from RLi and RMgX) Another important reactions: OH R H O oxidation H 1o alcohol reduction R C H aldehyde Ch. 12 - 6 Overall order H H C H H < H oxidation - 4 state lowest oxidation state of carbon H C H -2 O OH < H C 0 O H < H O C OH +2 < C O +4 highest oxidation state of carbon Ch. 12 - 7 3. Alcohols by Reduction of Carbonyl Compounds H R [H] O OH R OR' H OH (1o alcohol) [H] O O R R O R H [H] [H] R' HO R H R' Ch. 12 - 8 3A. Lithium Aluminum Hydride LiAlH4 (LAH) ● Not only nucleophilic, but also very basic ● React violently with H2O or acidic protons (e.g. ROH) ● Usually reactions run in ethereal solvents (e.g. Et2O, THF) ● Reduces all carbonyl groups Ch. 12 - 9 Examples O (1) R OH O OR' O R 2. H+, H2O H 2. H+, H2O H H OH R H H + HOR' OH 1. LiAlH4, Et2O (3) R 2. H+, H2O 1. LiAlH4, Et2O (2) R OH 1. LiAlH4, Et2O R H H Ch. 12 - 10 3B. Sodium Borohydride NaBH4 ● less reactive and less basic than LiAlH4 ● can use protic solvent (e.g. ROH) ● reduces only more reactive carbonyl groups (i.e. aldehydes and ketones) but not reactive towards esters or carboxylic acids Ch. 12 - 11 Examples O (1) R H O H2O R R' H2O H H OH NaBH4 (2) R OH NaBH4 R H R' Ch. 12 - 12 3C. Overall Summary of LiAlH4 and NaBH4 Reactivity reduced by LiAlH4 reduced by NaBH4 O O O < < R O O R OR' < R R' R H ease of reduction Ch. 12 - 13 5. Organometallic Compounds Compounds that contain carbon-metal bonds are called organometallic compounds C M primarily ionic (M = Na or K) C : M (M = Mg or Li) C M primarily covalent (M = Pb, Sn, Hg or Tl) Ch. 12 - 14 6. Preparation of Organolithium & Organomagnesium Compounds 6A. Organolithium Compounds Preparation of organolithium compounds R X + 2 Li Et2O (or THF) Order of reactivity of RX ● RI > RBr > RCl RLi + LiX Ch. 12 - 15 Example (80% - 90%) Et2O Br + 2 Li -10oC Li + LiBr Ch. 12 - 16 6B. Grignard Reagents Preparation of organomagnesium compounds (Grignard reagents) R Ar X X + + Mg Mg Et2O Et2O RMgX ArMgX Order of reactivity of RX ● RI > RBr > RCl Ch. 12 - 17 7B. Reactions of Grignard Reagents with Epoxides (Oxiranes) Grignard reagents react as nucleophiles with epoxides (oxiranes), providing convenient synthesis of alcohols RMgBr + O then H2O R OH Ch. 12 - 18 Via SN2 reaction R O R O H+, H2O R OH (1o alcohol) Ch. 12 - 19 Also work for substituted epoxides RMgBr + O H then H2O RMgBr + H (2o alcohol) R" R' OH R' R' O R then H2O R OH R" R' (3o alcohol) Ch. 12 - 20 7C. Reactions of Grignard Reagents with Carbonyl Compounds O + R R"MgX R' OH 1. Et2O 2. H3O + R R' R" R' = H (aldehyde) R' = alkyl (ketone) Ch. 12 - 21 Mechanism O + R R" O MgX R R' H OH R O MgX R" R' H H R" R' Ch. 12 - 22 8. Alcohols from Grignard Reagents O + R R"MgX R' OH 1. Et2O 2. H3O + R R' R" R' = H (aldehyde) R' = alkyl (ketone) Ch. 12 - 23 R, R’ = H (formaldehyde) o ● 1 alcohol R MgX O O MgX + R H H formaldehyde H H3O+ OH R H H H 1o alcohol Ch. 12 - 24 R = alkyl, R’ = H (higher aldehydes) o ● 2 alcohol R MgX O O MgX + R R' H higher aldehyde H H3O+ OH R R' R' H 2o alcohol Ch. 12 - 25 R, R’ = alkyl (ketone) o ● 3 alcohol R MgX O O + R R' R" ketone R' R" NH3Cl H2O OH R MgX R' R" 3o alcohol Ch. 12 - 26 Reaction with esters o ● 3 alcohol O + R OR' R"MgX OH 1. Et2O 2. H3O + R R" R" + R'OH Ch. 12 - 27 Mechanism O + R" R O MgX R OR' MgX OR' R" O + R'O R H OH R R" R" O H O H R MgX R" R" MgX R" R" Ch. 12 - 28 Examples MgBr (1) O Et2O + H OH H OMgBr H3O+ H H (1o alcohol) Ch. 12 - 29 Examples MgI (2) O Et2O + H3C H OH OMgI CH3 H3O+ CH3 H (2o alcohol) Ch. 12 - 30 Examples O (3) MgBr + Ph Ph OH (3o alcohol) Ph Et2O Ph H3O+ Ph Ph OMgBr Ch. 12 - 31 Examples O (4) MgI Et2O + Ph Ph OMe MgI OMgI O MgI OMe O Ph Ph OH H3O+ Ph (3o alcohol) Ch. 12 - 32 8A. How to Plan a Grignard Synthesis Synthesis of OH Me Me Ch. 12 - 33 Method 1 ● Retrosynthetic analysis OH MgBr Me Me O + Me Me disconnection ● Synthesis MgBr + Me OH O 1. Et2O + Me 2. H3O Me Me Ch. 12 - 34 Method 2 ● Retrosynthetic analysis OH Me Me MeMgBr O Me + disconnection ● Synthesis MeMgBr + O OH Me 1. Et2O 2. H3O+ Me Me Ch. 12 - 35 Method 3 ● Retrosynthetic analysis OH disconnection Me Me O OEt + 2 MeMgBr disconnection ● Synthesis O OEt + 2 MeMgBr OH 1. Et2O 2. H3O+ Me Me Ch. 12 - 36 8B. Restrictions on the Use of Grignard Reagents Grignard reagents are useful nucleophiles but they are also very strong bases It is not possible to prepare a Grignard reagent from a compound that contains any hydrogen more acidic than the hydrogen atoms of an alkane or alkene Ch. 12 - 37 A Grignard reagent cannot be prepared from a compound containing an –OH group, an –NH– group, an –SH group, a –CO2H group, or an –SO3H group Since Grignard reagents are powerful nucleophiles, we cannot prepare a Grignard reagent from any organic halide that contains a carbonyl, epoxy, nitro, or cyano (–CN) group Ch. 12 - 38 Grignard reagents cannot be prepared in the presence of the following groups because they will react with them: OH, NH2, SO3H, SH, O O C R, NO2, C OR, N, C H, O O H, CO2H, NHR, NH2, O Ch. 12 - 39 8C. The Use of Lithium Reagents R Li + O OLi R organolithium reagent aldehyde or ketone lithium alkoxide H3O+ OH R alcohol Organolithium reagents have the advantage of being somewhat more reactive than Grignard reagents although they are more difficult to prepare and handle Ch. 12 - 40 8D. The Use of Sodium Alkynides Preparation of sodium alkynides R NaNH2 H R -NH3 Reaction via ketones (or aldehydes) O R Na ONa Na + R OH H3O+ R Ch. 12 - 41 9. Protecting Groups HO I OH How? HO Ch. 12 - 42 Retrosynthetic analysis OH O HO MgBr + HO disconnection Br HO However Br HO Mg Et2O BrMg O MgBr H O acidic proton H powerful base Ch. 12 - 43 Need to “protect” the –OH group first HO Br (protection) OH "P"O "P"O Mg, Et2O O 1. 2. H3O+ Br "P"O MgBr (no acidic OH group) (deprotection) OH HO Ch. 12 - 44