Organometallic Reagents: Grignard & Organolithium Reactions
advertisement

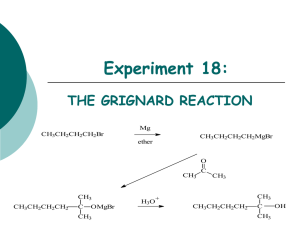
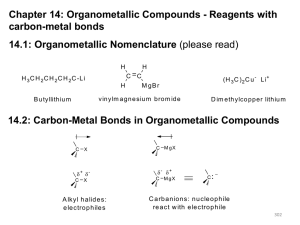
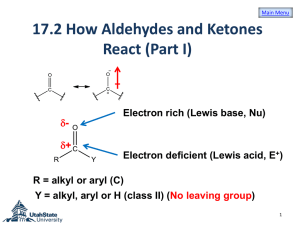
Organometallic Reagents Carbon-Metal Bonds + R – X – R + M Nucleophilic carbon Organometallic Reagents Organolithium Reagents normally prepared by reaction of alkyl halides with lithium R X + 2Li R Li + LiX same for Ar—X is an oxidation-reduction reaction: carbon is reduced Examples diethyl ether (CH3)3CCl + 2Li (CH3)3CLi + LiCl –10°C (75%) diethyl ether Br + 2Li Li + LiBr 35°C (95-99%) Preparation of Organomagnesium Reagents “Grignard” Reagents prepared by reaction of alkyl halides with magnesium R X + Mg RMgX same for Ar—X Diethyl ether is most often used solvent. Tetrahydrofuran is also used. Nobel Prize 1912 Born: 6 May 1871, Cherbourg, France Died: 13 December 1935, Lyon, France Affiliation at the time of the award: Nancy University, Nancy, France Examples diethyl ether Cl + Mg MgCl –10°C (96%) diethyl ether Br + Mg MgBr 35°C (95%) Alkyl halides, vinyl halides, and aryl halides can all be used to form organolithium and organomagnesium compounds However, these organometallic compounds cannot be prepared from compounds containing acidic groups (OH, NH2, NHR, SH, C=CH, CO2H) Cannot use H2O, CH3OH, CH3CH2OH, etc. as solvents Cannot prepare from substances such as HOCH2CH2Br, etc. Synthesis of Alcohols Grignard or Organolithium Reagents Organolithium reagents react with carbonyls in the same general way that Grignard reagents do. Grignard Reagents • Direct formation of a Grignard reagent, involves an alkyl halide reacted with Mg metal. Indirect Grignard Reagents Grignard Reagents can be prepared from other Grignard Reagents (trans-metallation) Indirect Grignard Reagents α-acetylenic Grignard Reagents are prepared by an acid-base Grignard reaction CH3CH2MgBr + HC CH stronger acid CH3CH3 weaker acid + HC CMgBr Question • Select the best method to prepare CH3CH2CCMgI. • A) CH3CH2CC-I + Mg (in diethyl ether) • B) I-CH2CH2CCH + Mg(OH)2 (in diethyl ether) • C) CH3CH2CCH + CH3MgI (in diethyl ether) • D) CH3CH2CCH + Mg (in diethyl ether) Grignard Reactions • A Grignard reagent, RMgX, is a very strong base and a good nucleophile; water/moisture MUST be avoided in its reaction. Question If water can’t be used as a solvent, can other protic solvents be used instead? Yes (A) / No (B) Example MgBr + CH3OH + (100%) CH3OMgBr Grignard reagents act as nucleophiles toward the carbonyl group – R + C diethyl ether R C • O • + MgX • •• •– MgX O •• •• H3O+ two-step sequence gives an alcohol as the isolated product R C •• OH •• Grignard reagents react with: formaldehyde to give primary alcohols other aldehydes to give secondary alcohols ketones to give tertiary alcohols esters to give tertiary alcohols Grignard reagents react with: formaldehyde to give primary alcohols Grignard reagents react with formaldehyde – R H + H C H diethyl ether R C •• O •• •• – MgX O •• •• H + MgX H3O+ product is a primary alcohol H R C •• OH •• H Example Mg Cl MgCl diethyl ether H C O H CH2OH (64-69%) H3O+ CH2OMgCl Grignard reagents react with: formaldehyde to give primary alcohols aldehydes to give secondary alcohols Question • The reaction between propanal and ethylmagnesium chloride followed by H3O+ will produce • A) B) • C) D) Grignard reagents react with aldehydes – R H + R' C MgX O •• •• H diethyl ether R C R' •• O •• + MgX •• – H3O+ product is a secondary alcohol H R C •• OH •• R' Example Mg CH3(CH2)4CH2Br diethyl ether CH3(CH2)4CH2MgBr H3C C O H CH3(CH2)4CH2CHCH3 OH (84%) H3O+ CH3(CH2)4CH2CHCH3 OMgBr Question • What is the product of the reaction of ethylmagnesium bromide (CH3CH2MgBr) with butanal (CH3CH2CH2CH=O) followed by dilute acid? • A) 2-hexanol • B) 1-butanol • C) 3-hexanol • D) 3-pentanol Example O H2 C CHLi + CH 1. diethyl ether 2. H3O+ CHCH OH (76%) CH2 Grignard reagents react with: formaldehyde to give primary alcohols aldehydes to give secondary alcohols ketones to give tertiary alcohols Grignard reagents react with ketones – R R" + R' C R" diethyl ether R C •• O •• •• – MgX O •• •• R' + H3O+ product is a tertiary alcohol MgX R" R C •• OH •• R' Question • What is the product of the reaction shown at the right? • A) B) • C) D) Example Mg CH3Cl HO (62%) CH3 diethyl ether H3O+ CH3MgCl O ClMgO CH3 Question • Two alcohols, each having the molecular formula C11H22O, are formed in the reaction of methyl lithium with 3-(R)-tertbutylcyclohexanone. These two alcohols are • A) constitutional isomers. • B) enantiomers in equal amounts. • C) enantiomers in unequal amounts. • D) diastereomers. Preparation of Tertiary Alcohols From Esters and Grignard Reagents Grignard reagents react with esters – R R' •• + OCH 3 •• C MgX O •• •• R' diethyl ether R C •• OCH3 •• • O • + MgX • •• •– but species formed is unstable and dissociates under the reaction conditions to form a ketone Grignard reagents react with esters – R R' •• + OCH 3 •• C R' diethyl ether R OCH3 •• • O • + MgX • •• •– MgX O •• •• this ketone then goes on to react with a second mole of the Grignard reagent to give a tertiary alcohol C •• –CH3OMgX R R' C O •• •• Example O 2 CH3MgBr + (CH3)2CHCOCH3 1. diethyl ether 2. H3O+ OH (CH3)2CHCCH3 CH3 (73%) Two of the groups attached to the tertiary carbon come from the Grignard reagent Question • Which one of the compounds below will be produced when 2 moles of methylmagnesium bromide is added to 1 mole of methyl propanoate (CH3CH2CO2CH3) followed by acid? • A) CH3CH2COCH3 • B) CH3CH2CO2-MgBr • C) (CH3)2C(OH)CH2CH3 • D) CH3CH2CH2CH3 • E) 3-methyl-2-butanol Question What is the product of the following reaction? O 2) H3O+ O A. 1) xs CH3CH2CH2MgBr, Et2O O C. O OH B. D. OH OH Grignard Reactions Question • Which one of the compounds below will be produced when methylmagnesium bromide is added to propanoic acid (CH3CH2CO2H)? • A) CH3CH2COCH3 • B) CH3CH2CO2-MgBr • C) CH3CH2CH(OH)CH3 • D) CH3CH2CH2CH3