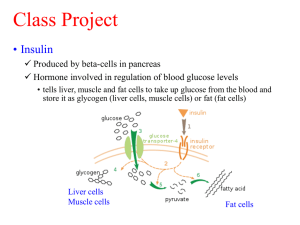
20131107Epac2A&AdipoRon
advertisement

Journal Club Takahashi T, Shibasaki T, Takahashi H, Sugawara K, Ono A, Inoue N, Furuya T, Seino S. Antidiabetic Sulfonylureas and cAMP Cooperatively Activate Epac2A. Sci Signal. 2013 Oct 22;6(298):ra94. Okada-Iwabu M, Yamauchi T, Iwabu M, Honma T, Hamagami KI, Matsuda K, Yamaguchi M, Tanabe H, Kimura-Someya T, Shirouzu M, Ogata H, Tokuyama K, Ueki K, Nagano T, Tanaka A, Yokoyama S, Kadowaki T. A small-molecule AdipoR agonist for type 2 diabetes and short life in obesity. Nature. 2013 Oct 30. doi: 10.1038/nature12656. 2013年11月7日 8:30-8:55 8階 医局 埼玉医科大学 総合医療センター 内分泌・糖尿病内科 Department of Endocrinology and Diabetes, Saitama Medical Center, Saitama Medical University 松田 昌文 Matsuda, Masafumi Glutamate Prentki M, Matschinsky FM, Madiraju SR.: Metabolic signaling in fuel-induced insulin secretion. Cell Metab. 18:162-85, 2013. sulfonylureas Glipizide Meglitinides (glinides) nateglinide Chlorpropamide Tolbutamide mitiglinide Acetohexamide Glibenclamide (glyburide) Tolazamide repaglinide Glimepiride Gliclazide http://www.jst.go.jp/pr/announce/20090731/ 1Division of Molecular and Metabolic Medicine, Kobe University Graduate School of Medicine, 7-5-1, Kusunoki-cho, Chuo-ku, Kobe 650-0017, Japan. 2Division of Diabetes and Endocrinology, Kobe University Graduate School of Medicine, Kobe 650-0017, Japan. 3Division of Cellular and Molecular Medicine, Kobe University Graduate School of Medicine, Kobe 650-0017, Japan. 4PharmaDesign Inc., 2-19-8, Hatchobori, Chuo-ku, Tokyo 104-0032, Japan. Sci Signal. 2013 Oct 22;6(298):ra94. BACKGROUND Sulfonylureas are widely used drugs for treating insulin deficiency in patients with type 2 diabetes. Sulfonylureas bind to the regulatory subunit of the pancreatic b cell potassium channel that controls insulin secretion. Sulfonylureas also bind to and activate Epac2A, a member of the Epac family of cyclic adenosine monophosphate (cAMP)–binding proteins that promote insulin secretion through activation of the Ras-like guanosine triphosphatase Rap1. MATERIALS AND METHODS 1 Reagents Glibenclamide, tolbutamide, chlorpropamide, acetohexamide, and glipizide were purchased from Sigma. Gliclazide was from LKT Laboratories Inc. [3H]Glibenclamide was from PerkinElmer. 8-pCPTwas from BioLog Life Science Institute. Molecular docking simulation and calculation of binding affinities MOE (CCG Inc.) and MOEASEDock (Ryoka Systems Inc.) (25) were used in docking studies of sulfonylureas to the active form of cNBD-A of Epac2A as well as in constructing the active form of cNBD-A of Epac2A. We constructed two types of cNBD-A models: one with the His124 side chain toward the inside of the pocket, and the other with the side chain toward the outside. We then used ASEDock to dock sulfonylureas against both cNBD-A models. For free energy calculations of sulfonylurea binding, we used the docked structures with His124-inside models. Using NAMD (43) for optimization of docked structures, we calculated the free energy of binding using solvated interaction energies (44). Cell culture and transfection MIN6-K8 b cells, Epac2A (Rapgef4)–deficient mouse clonal pancreatic b cells, and COS-1 cells were grown in Dulbecco’s modified Eagle’s medium containing 10% heat-inactivated fetal bovine serum and maintained in a humidified incubator with 95% air and 5% CO2 at 37℃ (12, 20, 24). Two days before FRETmeasurements, cells were transiently transfected with plasmids encoding FRET sensor, using Effectene Transfection Reagent (Qiagen). FRET experiments FRET experiments in MIN6-K8 b cells transfected with mouse wild-type Epac2A, mutant Epac2A, human wild-type Epac1 (45), or mutant Epac1 FRET sensor were performed as described previously (20). EYFP/ECFP ratio for Epac2A or EYFP/citrine ratio for Epac1 was normalized to R0 to describe FRET efficiency changes (FRET change = R/R0, where R0 is the ratio at time 0). FRET changes were acquired every 5 s. MATERIALS AND METHODS 2 Site-directed mutagenesis Site-directed mutagenesis was carried out with a KOD-PlusMutagenesis kit (Toyobo) according to the manufacturer’s instructions. Mutations were confirmed with an ABI 3700 PRISM (PerkinElmer Life Sciences) automated sequencer. Sulfonylurea binding experiments Sulfonylurea binding experiments were performed as described previously (20). Briefly, COS-1 cells transfected with Epac2A complementary DNA (cDNA) were resuspended in assay buffer containing 119 mM NaCl, 4.7 mM KCl, 2.5 mM CaCl2, 1.2 mMKH2PO4, 1.2 mMMgSO4, 5 mM NaHCO3, and 20 mM Hepes (pH 7.4). Each aliquot was incubated for 1 hour at room temperature with [3H]glibenclamide in the absence or presence of unlabeled glibenclamide at various concentrations. Bound [3H]glibenclamide was separated from free [3H]glibenclamide by rapid vacuum filtration through Whatman GF/C filters (Whatman International). The filters were washed with ice-cold buffer, and the radioactivity was determined with a liquid scintillation counter. Measurement of Rap1 activity Pull-down assay for Rap1-GTP (guanosine triphosphate) was performed as described previously (12). Dimethyl sulfoxide was used as vehicle. Precise quantification was achieved by densitometric analysis of the immunoreactive bands with the National Institutes of Health Image software. The intensity of the Rap1-GTP signal was normalized to that of total Rap1. Anti-Rap1 antibody was purchased from Millipore (07-916). . MATERIALS AND METHODS 3 Adenovirus-mediated gene transfer Adenovirus-mediated gene transfer was performed as described previously (20). Briefly, recombinant adenovirus carrying mCherry (Ad-mCherry) or Epac2A (Ad-Epac2A) was generated according to the manufacturer’s instructions (Invitrogen). Epac2A-deficient b cells were infected with Ad-mCherry or Ad-Epac2A. After 3 days of culture, the infected cells were preincubated with 2.8 mM glucose and then incubated with 2.8 mM glucose plus various stimuli for 15 min. GTP-bound Rap1 was assessed. Measurement of cAMP Measurement of cAMP was performed as described previously (20). Briefly, MIN6-K8 b cells were seeded at a density of 6 × 104 cells per well (96-well plate) and cultured for 2 days. After 30 min of preincubation with Krebs- Ringer bicarbonate buffer containing 2.8 mM glucose, the cells were treated with vehicle or adrenaline in the same buffer for 15min.The cellswere incubated, and cellular cAMP concentration was determined by HTRF (homogeneous time-resolved fluorescence assay) with theCisbio cAMPfemto 2 kit (Cisbio International) according to the manufacturer’s instruction. Epac2A Epac1 The cNBD of Epac1 binds cAMP with high affinity, whereas in Epac2A, cAMP binds to the cNBD-A, the first cNBD, with low affinity and to the cNBD-B, the second cNBD, with high affinity Fig. 1. Effects of 8-pCPT and sulfonylureas on Epac activation as revealed by FRET in MIN6K8 b cells. (A) FRET emission ratio time course of wild-type (WT) Epac2A exposed to the cAMP analog 8-pCPT. (B) FRET emission ratio time course ofWT Epac2A exposed to the indicated sulfonylurea at the indicated concentration. (C) FRET emission ratio time course of WT Epac1 exposed to the cAMP analog. (D) FRET emission ratio time course of WT Epac1 exposed to the indicated sulfonylurea at the indicated concentration. FRET change = R/R0. Data are presented as means ± SEM (n = 4 to 9 experiments for each point). Fig. 2. Structural model of the active form of the cNBD-A of Epac2A. (A) Diagram of the domain structures of Epac2A and Epac1. The regulatory region contains one or two cNBDs and a DEP domain. The catalytic region contains the REM, RA domain, and CDC25-HD. The numbers of the amino acid residues indicate the approximate domain boundaries within the primary structures. (B) Superimposition of the active form of cNBD-B in Epac2A and the inactive form of cNBD-A in Epac2A. Magenta, active form of cNBD-B in Epac2A (PDB ID: 3CF6); green, inactive form of cNBD-A in Epac2A (PDB ID: 1O7F). (C) Structural model of the active form of cNBD-A in Epac2A. We constructed a structural model of the active form of cNBD-A with the inactive form of cNBD-A (amino acid residues 44 to 139, PDB ID: 1O7F) and the active form of cNBD-B (amino acid residues 450 to 477, PDB ID: 3CF6). AMBER99 force field was applied for refinement of the structure. Yellow, b sheet; red, a helix. Labeled residues were predicted to interact with sulfonylureas. (D) Structural model of the active form of RA domain in Epac2A (PDB ID: 3CF6). The predicted amino acid residues that interact with sulfonylureas by docking simulation are shown in (C) and (D). Fig. 3. Predicted models of interactions of sulfonylurea and Epac2A. (A) Core structure of the sulfonylureas. (B) Model of the interaction between Epac2A cNBD-A and the inactive sulfonylurea. (C) Model of the interaction between Epac2A cNBD-A and sulfonylureas requiring a relatively high concentration to activate Epac2A. (D) Model of sulfonylureas requiring a relatively low concentration to activate Epac2A. (E) Model of the interaction between Epac2A cNBD-A and sulfonylurea. (F) Model of the interaction between Epac2A RA domain and sulfonylurea. (G) Predicted affinities for the sulfonylurea binding to Epac2A. Ball-and-stick indicates sulfonylureas; wire diagram indicates amino acids of Epac2A. Dotted lines indicate predicted interactions. Carbon, nitrogen, oxygen, and sulfur atoms are colored green, blue, red, and yellow, respectively. For tolbutamide, only those interactions subsequently confirmed by mutagenesis are shown. PDB files of models for the interactions between Epac2A and sulfonylureas are provided in the Supplementary Materials. Fig. 4. Effects of Epac mutations on Epac activation as revealed by FRET in MIN6K8 b cells. (A) FRET emission ratio time courses of WT Epac2A or mutant Epac2A in cells treated with the indicated activators. Data are presented as means ± SEM (n = 4 to 7 experiments for each point). (B) Alignment of the amino acid sequences of the cNBD-A of Epac2A and cNBD of Epac1. Residues predicted to be involved in sulfonylurea binding in Epac2A and the corresponding residues in Epac1 are in red. (C) FRET emission ratio time courses of triple-mutant Epac1 (T302C, L313S, and A322H) in cells treated with the indicated activators. The FRET response of WT Epac1 in response to 8-pCPT is shown for comparison. Data are presented as means ± SEM (n = 4 to 9 experiments for each point). Fig. 4. Effects of Epac mutations on Epac activation as revealed by FRET in MIN6K8 b cells. (A) FRET emission ratio time courses of WT Epac2A or mutant Epac2A in cells treated with the indicated activators. Data are presented as means ± SEM (n = 4 to 7 experiments for each point). (B) Alignment of the amino acid sequences of the cNBD-A of Epac2A and cNBD of Epac1. Residues predicted to be involved in sulfonylurea binding in Epac2A and the corresponding residues in Epac1 are in red. (C) FRET emission ratio time courses of triple-mutant Epac1 (T302C, L313S, and A322H) in cells treated with the indicated activators. The FRET response of WT Epac1 in response to 8-pCPT is shown for comparison. Data are presented as means ± SEM (n = 4 to 9 experiments for each point). Fig. 5. Dependence of Rap1 activation by glibenclamide on Epac2A. (A) Alignment of the amino acid sequences in the cNBD of Epac2A, Epac1, and PKA regulatory subunits. Asterisk indicates Ala-to-His substitution at position 124 in Epac2A. (B and C) Effects of glibenclamide and 8-pCPT on Rap1 activation in Epac2Adeficient b cells expressing mCherry (control) or exogenous WT Epac2A or Epac2A H124A mutant (H124A). Quantification of chemiluminescent signals is shown with corresponding bars positioned under the bands. Data are presented as means ± SEM (n = 4 to 7 experiments for each point). *P < 0.05; **P < 0.01; NS, not significant (Dunnett’s method). Fig. 6. cAMP-dependent activation of Epac2A and Rap1 by glibenclamide. (A to C) Graphs showing the direct binding of glibenclamide to Epac2A. [3H]Glibenclamide binding was assessed in COS-1 cells transfected with either WT Epac2A or Epac2A H124A. DPM, disintegration per minute. Data are presented as means ± SEM (n = 4 to 6 experiments for each point). Data are presented as means ± SEM (n = 4 to 7 experiments for each point). **P < 0.01; NS, not significant (Dunnett’s method). Fig. 6. cAMP-dependent activation of Epac2A and Rap1 by glibenclamide. (D) FRET emission ratio time courses of WT Epac2A in MIN6-K8 b cells exposed to the indicated compounds. (E) FRET emission ratio time courses of Epac2A H124A in MIN6-K8 b cells exposed to the indicated compounds. Data are presented as means ± SEM (n = 5 to 9 experiments for each point). Data are presented as means ± SEM (n = 4 to 7 experiments for each point). **P < 0.01; NS, not significant (Dunnett’s method). Fig. 6. cAMP-dependent activation of Epac2A and Rap1 by glibenclamide. (F and G) Rap1 activation assays after treatment with the indicated combinations of 8-pCPT and glibenclamide in MIN6-K8 b cells (F) or Epac2A-deficient b cells expressing mCherry (control) or exogenous WT Epac2A or Epac2A H124A (G). Data are presented as means ± SEM (n = 4 to 7 experiments for each point). **P < 0.01; NS, not significant (Dunnett’s method). Fig. 7. Effect of depletion of endogenous cAMP by adrenaline on the activation of Epac2A by sulfonylureas. (A and B) FRET emission ratio time courses of Epac2A in MIN6-K8 b cells treated with adrenaline and glibenclamide or tolbutamide. Time of application of the compounds is indicated by the bars. Data are presented as means ± SEM (n = 3 to 9 experiments for each point). Data are presented as means ± SEM (n = 6 experiments for each condition). ***P < 0.001. Statistical analysis was performed with Tukey’s method. Fig. 7. Effect of depletion of endogenous cAMP by adrenaline on the activation of Epac2A by sulfonylureas. (A and B) FRET emission ratio time courses of Epac2A in MIN6-K8 b cells treated with adrenaline and glibenclamide or tolbutamide. Time of application of the compounds is indicated by the bars. Data are presented as means ± SEM (n = 3 to 9 experiments for each point). Data are presented as means ± SEM (n = 6 experiments for each condition). ***P < 0.001. Statistical analysis was performed with Tukey’s method. Fig. 7. Effect of depletion of endogenous cAMP by adrenaline on the activation of Epac2A by sulfonylureas. (C) Rap1 activation assays in MIN6-K8 b cells treated with the indicated combinations of adrenaline and glibenclamide. Data are presented as means ± SEM (n = 6 experiments for each condition). ***P < 0.001. Statistical analysis was performed with Tukey’s method. SUMMARY Using molecular docking simulation, we identified amino acid residues in one of two cyclic nucleotide– binding domains, cNBD-A, in Epac2A predicted to mediate the interaction with sulfonylureas. We confirmed the importance of the identified residues by site-directed mutagenesis and analysis of the response of the mutants to sulfonylureas using two assays: changes in fluorescence resonance energy transfer (FRET) of an Epac2A-FRET biosensor and direct sulfonylurea-binding experiments. These residues were also required for the sulfonylurea dependent Rap1 activation by Epac2A. Binding of sulfonylureas to Epac2A depended on the concentration of cAMP and the structures of the drugs. CONCLUSION Sulfonylureas and cAMP cooperatively activated Epac2A through binding to cNBDA and cNBD-B, respectively. Our data suggest that sulfonylureas stabilize Epac2A in its open, active state and provide insight for the development of drugs that target Epac2A. Message Epac2Aについては再現性 が問題あるとされていた が、次第に確立されてき ているようである。 SU薬の結合はスルホニル 骨格とベンザミド骨格が SURの細胞の内側部位にそ れぞれ結合するとされる。 今回Epac2Aにスルホニル 骨格が結合しインスリン 分泌を促すことが示され た! グリニド薬はベンザミド 骨格しかないが、更に、 Epac2Aにも結合しない。 SU薬の標的は、これまではSU受容体が唯一 知られており、SU薬によるインスリン分泌は SU受容体を介するメカニズムのみで説明され ていた(青部分)。本研究によりSU薬の作用に は、Epac2/Rap1を介するメカニズムも重要で あることが解明された(赤部分)。Epac2はイン クレチンによるインスリン分泌の増強にも重要 であることが示されている。 要約:2型糖尿病患者におけるインスリン欠乏の治療には、スルホニル尿素 が広く使用されている。スルホニル尿素は、インスリン分泌を制御している膵 β細胞のカリウムチャネルの調節サブユニットに結合する。またスルホニル尿 素は、Ras様グアノシントリホスファターゼRap1の活性化を介してインスリン 分泌を促進している、環状アデノシン一リン酸(cAMP)結合タンパク質の EpacファミリーのメンバーであるEpac2Aにも結合し、活性化している。 われわれは分子ドッキングシミュレーションを用い、Epac2Aの2つの環状ヌク レオチド結合ドメインのうち1つ(cNBD-A)に、スルホニル尿素との相互作用 を媒介すると予測されるアミノ酸残基を特定した。さらに、この特定された残 基の重要性を、部位特異的変異導入法と、以下の2つのアッセイを用いたス ルホニル尿素に対する突然変異体の反応の解析により確認した。すなわち、 Epac2A-FRETバイオセンサーの蛍光共鳴エネルギー移動(FRET)の変化、 および直接のスルホニル尿素結合実験である。 これらの残基は、Epac2Aによるスルホニル尿素依存性のRap1活性化にも 必要であった。Epac2Aに対するスルホニル尿素の結合は、cAMP濃度と薬 物の構造に依存していた。スルホニル尿素とcAMPは、それぞれcNBD-Aと cNBD-Bに結合することでEpac2Aを協調的に活性化していた。われわれの データは、スルホニル尿素がEpac2Aを開放型の活性化状態で安定化するこ とを示唆し、さらにEpac2Aを標的とする薬物の開発の手がかりを示している。 Fig. 1 Schematic representation of known and assumed pathways for adiponectin signal transduction. Adiponectin receptor 1 (AdipoR1) has a high affinity for globular adiponectin and a low affinity for full-length adiponectin, whereas adiponectin receptor 2 (AdipoR2) has an intermediate affinity for fulllength and globular adiponectin. T-cadherin is a truncated receptor that can bind the hexameric and high molecular weight (HMW) oligomeric forms of adiponectin. AdipoR1 and AdipoR2 interact with the adaptor protein containing a pleckstrin homology domain, a phosphotyrosine domain and a leucine zipper motif (APPL1), which binds the N-terminal intracellular domains of the receptors. The binding of adiponectin to its receptors provokes the activation of adenosine monophosphate (AMP)-activated protein kinase (AMPK), and the activation of various signaling molecules such as p38 mitogen-activated protein kinase (p38 MAPK), peroxisome proliferator-activated receptor-α (PPARα), the RAS-associated protein Rab5, phosphatidylinositol 3-kinase (PI3K) and the vakt murine thymoma viral oncogene homolog (Akt). Activation of AMPK can also block the nuclear factor κ B (NFκB) signaling, known to be a mediator of inflammation in endothelial cells. ACC acetyl coenzyme-A carboxylase; Brochu-Gaudreau K, Rehfeldt C, Blouin R, Bordignon V, Murphy BD, Palin MF.: Adiponectin action from head to toe. Endocrine. 2010 Feb;37(1):11-32. Figure 2 Regulation of adiponectin transcription by upstream signals In obesity, increased pro-inflammatory cytokines such as TNFα, IL-6 and IL-18 negatively regulate adiponectin gene transcription by activating several pathways such as the JNK and ERK1/2 pathways. High-fat-diet (HFD)-induced obesity also suppresses adiponectin expression by increasing cellular levels of catecholamine and PKA-mediated activation of CREB [85]. Insulin has been suggested to positively regulate adiponectin gene expression by activating PPARγ via suppressing FoxO1 activity in vitro [50]; however, a negatively correlated relationship has been documented between insulin and adiponectin levels in vivo (see the text). FoxO1 could have a positive effect on adiponectin transcription via interaction with C/EBP [49,169]. Regulation of FoxO1 by insulin and Sirt1 may provide a mechanism to dynamically regulate adiponectin gene expression. IR, insulin receptor; IRS, insulin receptor substrate; P-FoxO1, phosphorylated FoxO1; PI3K, phosphoinositide 3-kinase; RXR, retinoid X receptor; TNFR-1, TNFα receptor 1. An animated version of this Figure can be seen at http://www.BiochemJ.org/bj/425/0041/bj4250041add.htm 1Department of Diabetes and Metabolic Diseases, Graduate School of Medicine, The University of Tokyo, Tokyo 113-0033, Japan. 2Department of Integrated Molecular Science on Metabolic Diseases, 22nd Century Medical and Research Center, The University of Tokyo, Tokyo 113-0033, Japan. 3Department of Molecular Medicinal Sciences on Metabolic Regulation, 22nd Century Medical and Research Center, The University of Tokyo, Tokyo 113-0033, Japan. 4RIKEN Systems and Structural Biology Center, Tsurumi, Yokohama 230-0045, Japan. 5Graduate School of Comprehensive Human Sciences, University of Tsukuba, Tsukuba 305-8577, Japan. 6Open Innovation Center for Drug Discovery, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-0033, Japan. 7Graduate School of Science, The University of Tokyo, Bunkyo-ku, Tokyo 113-0033, Japan. Background Adiponectin secreted from adipocytes binds to adiponectin receptors AdipoR1 and AdipoR2, and exerts antidiabetic effects via activation of AMPK and PPAR-a pathways, respectively. Levels of adiponectin in plasma are reduced in obesity, which causes insulin resistance and type 2 diabetes. Thus, orally active small molecules that bind to and activate AdipoR1 and AdipoR2 could ameliorate obesity-related diseases such as type 2 diabetes. Content Here we report the identification of orally active synthetic small-molecule AdipoR agonists. One of these compounds, AdipoR agonist (AdipoRon), bound to both AdipoR1 and AdipoR2 in vitro. Extended Data Figure 1 | Phosphorylation of AMPK in C2C12 myotubes. Phosphorylation of AMPK normalized to the amount of AMPK in C2C12 myotubes treated for 5 min with 15 μgml-1 adiponectin or the indicated small molecule compounds (10 mM). #, AdipoRon; ##, no. 112254; ###, no. 165073. Figure 1 | Smallmolecule AdipoR agonist AdipoRon binds to both AdipoR1 and AdipoR2, and increases AMPK activation, PGC-1a expression and mitochondrial biogenesis in C2C12 myotubes. Figure 1 | Small-molecule AdipoR agonist AdipoRon binds to both AdipoR1 and AdipoR2, and increases AMPK activation, PGC-1a expression and mitochondrial biogenesis in C2C12 myotubes. a, Chemical structure of AdipoRon. b–i, l, m, Phosphorylation and amount of AMPK (b–f, l,m), Ppargc1amRNAlevels (g, h), and mitochondrial content as assessed by mitochondrial DNA copy number (i), in C2C12 myotubes after myogenic differentiation (b–i), in skeletal muscle (l) or in liver (m) from wild-type (WT) or Adipor1-/- Adipor2 -/double-knockout mice, treated with indicated concentrations of AdipoRon (b, d–i) or adiponectin (d, 15μgml -1; e, 50μgml-1; i, 10μgml -1), for 5 min (b, d–f), 1.5 h (g, h) and 48 h (i), with or without EGTA (f, h), 25 μM AdipoRon, compound 112254 and 165073, 30 μgml-1 adiponectin for 5 min or 1mMAICAR for 1 h and transfected with or without the indicated siRNA duplex (c), or AdipoRon (l, m). j, k, Surface plasmon resonance measuring AdipoRon binding to AdipoR1 and AdipoR2. AdipoR1 and AdipoR2 were immobilized onto a sensor chip SA. Binding analyses were performed using a range of AdipoRon concentrations (0.49– 31.25 μM). All values are presented as mean6s.e.m. b, c, e–I, n=4 each; d, l,m, n=3 each; *P<0.05 and **P<0.01 compared to control or unrelated siRNA or as indicated. NS, not significant. Figure 2: AdipoRon improved insulin resistance, glucose intolerance and dyslipidaemia via AdipoR. Figure 2: AdipoRon improved insulin resistance, glucose intolerance and dyslipidaemia via AdipoR. Figure 2: AdipoRon improved insulin resistance, glucose intolerance and dyslipidaemia via AdipoR. a–g, Plasma AdipoRon concentrations (a), body weight (b), food intake (c), plasma glucose (d, e, g), plasma insulin (d, e) and insulin resistance index (f) during oral glucose tolerance test (OGTT) (1.0 g glucose per kg body weight) (d, e) or during insulin tolerance test (ITT) (0.5 U insulin per kg body weight) (g) in wild-type (WT) and Adipor1−/− Adipor2−/− double-knockout mice, treated with or without AdipoRon (50 mg per kg body weight). h, i, Glucose infusion rate (GIR), endogenous glucose production (EGP) and rates of glucose disposal (Rd) during hyperinsulinaemic euglycaemic clamp study in wild-type and Adipor1−/− Adipor2−/− double-knockout mice, treated with or without AdipoRon (50 mg per kg body weight). j, k, Plasma triglyceride (j) and free fatty acid (FFA) (k) in wild-type and Adipor1−/− Adipor2−/− double-knockout mice, treated with or without AdipoRon (50 mg per kg body weight). All values are presented as mean ± s.e.m. a, n = 12–32; b–g, j, k, n = 10 each; h, i, n = 5 each; *P < 0.05 and **P < 0.01 compared to control or as indicated. NS, not significant. Figure 4: AdipoRon ameliorated insulin resistance, diabetes and dyslipidaemia in db/db mice. a, Plasma glucose levels after intraperitoneal injection of adiponectin (30 µg per 10 g body weight) (left) or after oral administration of AdipoRon (50 mg per kg body weight) (middle). The area under the curve (AUC) of left and middle panels is shown on the right. b–i, Body weight (b), food intake (c), liver weight (d), WAT weight (e), treated with or without AdipoRon (50 mg per kg body weight). All values are presented as mean ± s.e.m. a, n = 6-7; b–i, n = 10 each from 2–3 independent experiments, *P < 0.05 and **P < 0.01 compared to control or as indicated. NS, not significant. Figure 4: AdipoRon ameliorated insulin resistance, diabetes and dyslipidaemia in db/db mice. plasma glucose (f, left, g), plasma insulin (f, middle) and insulin resistance index (f, right) during oral glucose tolerance test (OGTT) (1.0 g glucose per kg body weight) (f) or during insulin tolerance test (ITT) (0.75 U insulin per kg body weight) (g), plasma triglyceride (h) and free fatty acid (FFA) (i) in db/db mice under normal chow conditions, treated with or without AdipoRon (50 mg per kg body weight). All values are presented as mean ± s.e.m. a, n = 6-7; b–i, n = 10 each from 2–3 independent experiments, *P < 0.05 and **P < 0.01 compared to control or as indicated. NS, not significant. Figure 5: AdipoRon increased mitochondria biogenesis in muscle, reduced tissue triglyceride content and oxidative stress in muscle and liver, and decreased inflammation in liver and WAT of db/db mice. a–h, Ppargc1a, Esrra, Tfam, mt-Co2, Tnni1, Acadm and Sod2 mRNA levels (a), and mitochondrial content as assessed by mitochondrial DNA copy number (b), tissue triglyceride content (c) and TBARS (d) in skeletal muscle, Ppargc1a, Pck1, G6pc, Ppara, Acox1, Ucp2, Cat, Tnf and Ccl2 mRNA levels (e), tissue triglyceride content (f) and TBARS (g) in liver, and Tnf, Il6, Ccl2, Emr1, Itgax and Mrc1 mRNA levels (h) in WAT from db/db mice on a normal chow diet, treated with or without AdipoRon (50 mg per kg body weight). All values are presented as mean ± s.e.m. n = 10, *P < 0.05 and **P < 0.01 compared to control or as indicated. NS, not significant. Figure 6: AdipoRon increased insulin sensitivity and glucose tolerance, and at the same time contributed to longevity of obese diabetic mice. a–c, Kaplan–Meier survival curves for wild-type, Adipor1−/−, Adipor2−/− and Adipor1−/− Adipor2−/− knockout mice on a normal chow diet (a) (n = 50, 32, 29 and 35, respectively) or high-fat diet (b) (n = 47, 33, 35 and 31, respectively), or for db/db mice treated with or without AdipoRon (30 mg per kg body weight) on a normal chow or high-fat diet (n = 20 each) (c). P values were derived from logrank calculations. d, Scheme illustrating the mechanisms by which AdipoR1 and AdipoR2 agonist increases insulin sensitivity and glucose tolerance, and at the same time lifespan. Summary AdipoRon showed very similar effects to adiponectin in muscle and liver, such as activation of AMPK and PPAR-a pathways, and ameliorated insulin resistance and glucose intolerance in mice fed a high-fat diet, which was completely obliterated in AdipoR1 and AdipoR2 double-knockout mice. Moreover, AdipoRon ameliorated diabetes of genetically obese rodent model db/db mice, and prolonged the shortened lifespan of db/db mice on a high-fat diet. Conclusion Thus, orally active AdipoR agonists such as AdipoRon are a promising therapeutic approach for the treatment of obesityrelated diseases such as type 2 diabetes. Message 10月29日,東京大学病院糖尿病・代謝内科教授の門脇 孝氏,同科講師の山内敏正氏らは記者会見を開き,同氏 らの研究グループが発見した,アディポネクチン受容体 を活性化させる低分子化合物に関する研究成果について 発表した。2003年にアディポネクチン受容体を同定した 同氏らは,今回,肥満によって発現が減少する同受容体 を活性化させる働きを持つ化合物AdipoRonを同定。マウ スを用いた実験により,糖尿病をはじめとする生活習慣 病の予防や治療,健康寿命の延長につながる経口薬の種 となる可能性が高いとして,今後5年以内の臨床第Ⅰ相 試験を視野に創薬を目指すと述べた。なお,研究の詳細 は,Nature(2013年10月30日オンライン版)に掲載され た。 http://mtpro.medical-tribune.co.jp/mtpronews/1310/1310084.html