Amines Powerpoint
advertisement

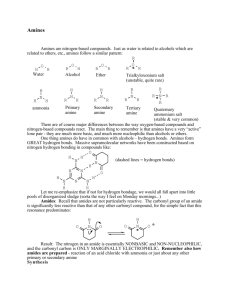
Amines By: David Eastwood & Morgan Gentes What is an Amine? Amines are found everywhere. They appear mainly in decaying animal and plant matter. Amines are what give off a pungent odour, mainly being the one we recognize as decaying flesh. Ironically, amines are also are huge part of the compositions of perfumes and colognes. Animals are attracted to the scent of decaying flesh. Apparently so are humans. Structure Amines are a derivative of ammonia where one or more hydrogen atoms are replaced by an substituent. cyclobutyl amine or cyclobutanamine Nomenclature When naming Amines, use the prefix “amino” and the suffix “amine”. Lower amines are named with the suffix amine. H H \ | N–C–H / | H H Methylamine Since we know that the more oxidized (bonds to oxygen) group is the principal group, in some cases in may not be the suffix amine used but the prefix “amino”. In other situations, H2N OH \ / C-C-C-C 3-amino-1-propanol RNH2 Primary Amine R2NH Secondary Amine R3N Tertiary Amine Ammonia VS. Amine The chemistry of amines and ammonia are similar. The most obvious feature is their basicity (being able to accept a proton) which comes from the unshared pair of electrons in the nitrogen atom. Comparing acceptance of a proton in an Amine and an ammonia is shown: H R\ | R- N : + H R – N – H R/ | R H R\ | R- N : + H R – N – H R/ | R Drawing amines is just as simple as naming them. “N” represents a locator, just like numbers. Ex. N-ethyl-3-methyl-1-pentanamine CH3 N-CH2-CH3 l / CH3-CH2-CH-CH2-CH Draw 1-amino-2-methyl-5-octene-7-ol OH NH2 \ / CH2-CH-C=C-CH-CH2-CH-CH2 | CH3 Methylamine http://upload.wikimedia.org/wikipedia/commons/archive/7/7f/20100103095327!Methylamine-2D.png ethyl amine or ethanamine 3-amino-2,6-dimethyl-4propyloctane Prepared Amines Amines can be prepared by a substitution process called Alkylation, where an alkyl group becomes bonded to a Nitrogen. http://www.chem.ucalgary.ca/courses/351/Carey5th/Ch22/ch22-3-1.html Reactions References www.chem.ucalgary.ca/courses/350/Carey/.../amines/amin es-1.html ^ McMurry, John E. (1992), Organic Chemistry (3rd ed.), Belmont: Wadsworth, ISBN 0-534-16218-5 ^ Lide, D. R., ed. (2005), CRC Handbook of Chemistry and Physics (86th ed.), Boca Raton (FL): CRC Press, ISBN 0-8493-0486-5 ^ Karsten Eller, Erhard Henkes, Roland Rossbacher, Hartmut Höke "Amines, Aliphatic" in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2005. doi:10.1002/14356007.a02_001 ^ March, Jerry (1992), Advanced Organic Chemistry: Reactions, Mechanisms, and Structure (4th ed.), New York: Wiley, ISBN 0-471-60180-2