Naming Ionic Compounds
advertisement

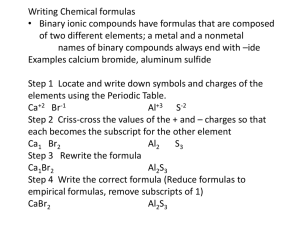
Sec. 2.2 Part A Science 10 NAMING IONIC COMPOUNDS REVIEW How do atoms form ions? Why do they want to form ions? What are positively charged ions called? Negatively charged ions? What can occur once ions have been created? Why does this occur? OBJECTIVES explain why the IUPAC system of naming compounds is important describe the process of ionic bonding and give examples of ionic compounds give correct names and formulas for ionic compounds, using the periodic table, table of ions and IUPAC rules IUPAC AND COMPOUND NAMING IUPAC – International Union of Pure and Applied Chemistry Responsible for naming compounds Allows consistent way of naming compounds Why is this important? Allows for clear and precise communication IONIC COMPOUNDS How do we form ionic compounds? Transfer of electrons between atoms Metal and non-metal Transfer of electrons creates full outer energy levels More stable Attraction of ions called ionic bonding IONIC COMPOUNDS Practice: Give drawings and show electron transfer for the following atoms: Sodium Magnesium Chlorine Oxygen NAMING IONIC COMPOUNDS: THE RULES Two part name: cation and anion 1) name cation first with element’s name 2) name anion second with first part of name and change last part to “ide” Ex. Sodium chloride (sodium ion and chloride ion) IMPORTANT: always write names of elements as lower case (unless at beginning of sentence) PRACTICE Name the following compounds: MgO BaF2(s) K3N(s) FORMULAS FOR IONIC COMPOUNDS Contains element symbols Some have subscripts Ex. BaF2(s) What this means: One barium ion for every two fluoride ions Ionic compounds are neutral Therefore, need to have enough of each atom to cancel the charges out FORMULAS FOR IONIC COMPOUNDS What are the charges for each ion in this compound? Sodium chloride How many of each ion do we need to cancel the charges out? How about this one? Magnesium chloride How many do we need? These make the subscripts! STEPS FOR WRITING FORMULAS Steps Example Example Sodium chloride Aluminium chloride Identify ions and charges sodium: Na+ chloride: Cl- aluminium: Al3+ chloride: Cl- Determine total charges needed to balance Na+ : 1 Cl- : 1 Al3+ : 3 Cl- : 1+1+1=3 Note the ratio of cations to anions 1 to 1 1 to 3 Use subscripts to write formula NaCl AlCl3 If the ratio is 1:1, do not need to include subscripts The subscripts should be the simplest form What does this mean? Formula unit- name for ionic compound unit (NEUTRAL) LOWEST COMMON MULTIPLE When charges are not easy to balance: i.e. calcium nitride Ca2+ and N3 Find the lowest common multiple to balance the charges What is the lowest common multiple here? Simplify!!! What do you end up with? BALANCING CHARGES Need to balance the charges i.e. one positive charge balances out one negative charge Why do we need to balance the charges? What happens to our compounds when we do this? PRACTICE Write the formulas/names for the following compounds: sodium bromide calcium nitride magnesium oxide aluminium chloride MgS AlN Li3P MULTIVALENT ELEMENTS Some metals have more than one stable ion To indicate which ion it is, use Roman numerals in names Ex. Iron has two stable ions: Fe2+ and Fe3+. Example: iron (II) or iron (III) Still use subscripts for compounds- be careful about which ion it is! Ex. FeBr2 = iron (II) bromide Ex. FeBr3 Only use Roman numerals when more than one ion (ONLY for transition metals) PRACTICE Write formulas for following: copper (I) nitride lead (IV) chloride nickel (III) oxide Write the names for the following formulas: AuN CrO TiBr4 Sec A2.2 Part B Science 10 NAMING IONIC AND MOLECULAR COMPOUNDS REVIEW How does an ionic compound form? What name would you give for the following? MgCl2 LiBr K3N FeCl3 OBJECTIVES predict formulas and write names for ionic compounds with polyatomic ions describe covalent bonding in molecular compounds identify diatomic/polyatomic molecular elements give correct names and formulas for molecular compounds with and without hydrogen using periodic table and IUPAC rules POLYATOMIC IONS Polyatomic ions- ions made of several nonmetallic atoms Come as one “unit” (consider to be one unit when naming) List in periodic table of polyatomic ions with symbol and charge Ending usually “-ate” (more oxygen) or “-ite” (less oxygen) NAMING WITH POLYATOMIC IONS Cation + anion DO NOT change the ending of a polyatomic ion Practice: Au(NO3)3(s) (NH4)3PO4(s) K2Cr2O7(s) WRITING FORMULAS Same method with exception: Subscripts for polyatomic ions placed in brackets Ex. Fe2(SO4)3 Put 3 outside brackets because there are 3 SO42- for each Fe3+ Practice: barium hydroxide iron (III) carbonate copper (I) permanganate MOLECULAR COMPOUNDS Molecule: two or more non-metal atoms bonding Can be same or different atoms Fixed numbers of bonded atoms (unlike ionic compounds: formula unit= part of crystal lattice) COVALENT BONDS How molecular compounds bond Atoms share electrons (no transfer) Pair of shared electrons makes one covalent bond Allows outer energy levels to be filled Can share more than one pair of electrons (double or triple bonds) http://www.youtube.com/watch?v=QqjcCvzWwww MOLECULAR ELEMENTS Elements that form own molecules (ex. O2) Diatomic- molecule made of 2 of the same atom H2, N2, O2, F2, Cl2, Br2, I2. Polyatomic- More than two of the same atom O3, P4, S8. Only appear as these when by themselves Should memorize these! NAMING MOLECULAR COMPOUNDS WITHOUT HYDROGEN For binary compounds (two elements) Use Greek prefixes: What are they for numbers 1-10? Indicates how many of each atom Prefix + first element followed by prefix + second element ending in “-ide” Ex. N2O (dinitrogen monoxide) PBr3 CO CS2 PRACTICE Write the names or formulas for the following: CO2(g) N2O(g) PCl3(g) oxygen difluoride dinitrogen tetrasulfide sulfur trioxide NAMING MOLECULAR COMPOUNDS WITH HYDROGEN Often given names Ex. “water” official IUPAC name for H2O Table A2.12 (Be familiar with this chart! Should know the important ones/ones you will see most often) HOW DO WE TELL THE DIFFERENCE? Go through difference between ionic and molecular compounds How do we know which is which and when to use what naming rules (dichotomous key)