Ionic Compounds and Naming
advertisement

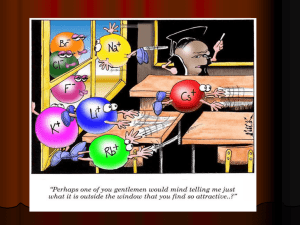
Ionic Compounds and Naming Chapter 4.10,4.11 and 5 When we begin combining elements we make compounds. Two types ◦ Non-metal and metal ◦ Non-metal and non-metal Each type has unique physical and chemical properties Look at non-metal and metal in detail now and non-metal and non-metal later Combining Elements A metal and a non-metal combination make an ionic compound ◦ Each element or polyatomic group is an ion ◦ What makes an ion? An ion is a charged particle (positive or negative) Difference between protons and electrons in an element ◦ Ca vs Ca+2 (Ca+2 has 2 less electrons than Ca) Ionic compounds The number of valence electrons determines the type of ion formed ◦ Trying to reach noble gas state Lose or gain electrons to reach magic number 8 ◦ Valence electrons are known by group number Main group elements only These atoms follow a pattern down the column Valence electrons vs charge How many valence electrons do the following elements have? What would their charge be? ◦K ◦P Mg O Al Br C Ar ◦ Do you see any pattern to the charges? Metals make positive ions (cations) Non-metals make negative electrons (anions) Practice First determine the charge on the cation and the anion. Then adjust the number of cations and anions you need to make the compound neutral. All ionic compounds are overall neutral; that is when you add up all the charges the sum is zero Making ionic compounds NaCl is made with Na+1 and Cl-1 MgO is made with Mg+2 and O-2 Na2O is made with 2 Na+1 and O-2 MgCl2 is made with Mg+2 and 2 Cl-1 Making ionic compounds Combine K and F to make a neutral compound Put the following elements together to make ionic compounds Be and F Li and S Ba and N K and As Cs and C ◦ ◦ ◦ ◦ K makes a K+1 ion F makes a F-1 ion Need one of each to make the ionic compound neutral KF Practice What about the elements in-between group II and III? ◦ Called transition metals because they can make more than one positive charge ◦ On our tables you can tell by the small black number above the symbol….. Transition metals Cu II and S W IV and O Pd II and N Cr III and Si Practice with transition metals Some ions are actually a group of elements combined together ◦ Can not be broken apart ◦ Act as one unit These are known as polyatomic ions ◦ See handout Polyatomic Ions Naming Binary Compounds We can easily name binary compounds both ionic and covalent (non-metals) ◦ Non-metals and metals use type I and II ◦ Non-metals and non-metals use type III Naming Binary Compounds Type I ◦ Metal bonded to non-metal ◦ Metal always listed and named first No changes in the metal name ◦ Metals only make ONE ion (known as simple metals) ◦ Anion is listed second Use the root of the name and add –ide ◦ Hydrogen becomes hydride ◦ Halogens remove –ine and add –ide ◦ Oxide, nitride, sulfide ◦ NaCl is sodium chloride Naming Binary Ionic Compounds Type II Cation is first again and anion is changed the same as type I The difference is that we need to designate the charge of the transition metal with a Roman Number USE THE ANION TO DETERMINE THE CHARGE OF THE CATION!!! ◦ use transition metals CuO ◦ ◦ ◦ ◦ Oxygen is -2 The compound must be neutral So the copper (Cu) must be +2 The compound is named copper II oxide Name the following ionic compounds: CaF AlCl3 MgI2 CuBr2 Al2O3 CrCl3 Fe2O3 FeO FeCl3 The number of atoms has no influence on the name for type I and II Practice Polyatomic ions just use their name K2SO4 would be potassium sulfate NH4NO3 would be ammonium nitrate Co(NO2)2 (NH4)3N Polyatomic Ions Type III ◦ Non-metal to non-metals NUMBER OF ATOMS IS IMPORTANT FOR TYPE III Use prefixes to determine the number of atoms in the name Same naming scheme as type I Use entire name for 1st element -ide for 2nd Add prefixes for multiple atoms ◦ Note: mono- is never used on 1st element Prefixes ◦ ◦ ◦ ◦ ◦ ◦ ◦ ◦ ◦ ◦ 1 – mono 2 – di 3- tri 4- tetra 5- penta 6- hexa 7- hepta 8- octa 9- nona 10- deca CCl4 would be carbon tetrachloride N2O2 would be dinitrogen dioxide PCl5 P4O6 N2O5 SF6 CO NO2 Practice Acids are a special group of binary compounds and have their own naming rules. All acids begin with H and are dissolved in water Acids without oxygen ◦ Use root of anion and add –ic and acid ◦ HCl is hydrochloric acid Acids with oxygen ◦ ◦ ◦ ◦ -ite becomes –ous -ate becomes –ic H2SO3 is sulfurous acid H2SO4 is sulfuric acid Naming Acids If the anion ends in –ide ◦ Start with hydro ◦ Use the root of the anion ◦ Add –ic acid to the end If the anion ends in –ate ◦ NO HYDRO ◦ Use the root or slightly more and add –ic acid If the anion ends in –ite ◦ NO HYDRO ◦ Use the root or slightly more and add –ous acid Naming acids Name these acids HF HNO3 H3PO4 Practice Write the formulas from these names: Nitric acid Potassium sulfide Sodium carbonate Dinitrogen pentoxide Reversing the process