Ionic and Covalent Bonding - Fall River Public Schools
advertisement

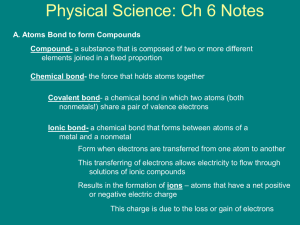
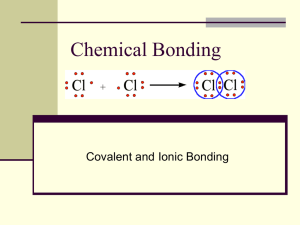
The Chemical Bond Ionic and Covalent Bonds Chemistry Ms. Piela Ionic Compounds Overview Bonds formed between a metal and a nonmetal Metals form positive ions called cations Trick to remember: “ca+ion” Non-metals form negative ions called anions Ionic bonds are formed by a transfer of electrons Properties of Ionic Compounds Ionic compounds dissociate (or break apart) in liquids Example: Table salt in water Ionic compounds can conduct electricity If something conducts electricity, then ions/charges must be able to move Solid ionic compounds are rigid, not allowing charges to move Two conditions allow ionic compounds to conduct electricity: Molten (or liquid) Dissolved in solution Example: Li-ion batteries Properties of Ionic Compounds Ionic compounds tend to have high melting points This is due to the high strength of the ionic bonds Stronger bonds need more energy to break apart! Formation of Ionic Compounds Ionic bonds follow the Octet Rule Octet rule – Each element tends to form compounds based on the easiest way to gain 8 valence electrons Example: Na and Cl Sometimes combinations are not an easy one-to-one ratio! Example: Ca and Cl Formation of Ionic Compounds Examples Mg and O K and S Practice Ba and N Li and P Formation of Ionic Compounds Transition metal ionic compounds All form positive ions (cations), but can form multiple ions Example: FeCl2 and FeCl3 Covalent Compounds Overview Covalent bonds are made between nonmetals only Does NOT depend on charges!! Covalent bonds will share electrons as both elements need to gain electrons to obtain full outer shell (8 valence electrons) Properties of Covalent Compounds Forms longer bonds because bonds are weaker than ionic bonds Energy is lowered when atoms form a covalent bond This is the main driving force behind their formation There is a balance between their attractive and repulsive forces Two Types of Covalent Bonds Polar covalent bonds are covalent bonds where electrons are shared unequally between the atoms Non-polar covalent bonds are covalent bonds where electrons are shared equally between the atoms Polarity Polarity depends on differences in electronegativity The greater the difference in electronegativity, the more polar the bond is Example: Cl2 and HF The more polar the bond, the stronger the bond Polar covalent bonds have stronger bonds than non-polar bonds Stronger bonds give higher boiling points (just like ionic compounds) The Chemical Bond Overview In order of increasing bond strength: In order of increasing bond length: Naming Ionic Compounds Simple Naming Rules Identify the compound as Ionic (Metal and a non-metal) Determine charges for each ion Overall charge of compound must be zero General formula for names: Cation + Anion Root-ide Examples Calcium fluoride Calcium phosphide Magnesium nitride Potassium oxide Examples LiCl2 SrBr2 BaI2 Transition Metal Compounds Since transition metals form multiple ions, roman numerals denote charge Examples iron (III) chloride copper (II) oxide iron (III) oxide silver (I) sulfide Polyatomic Ion Compounds Some elements are considered as a charged group of two or more atoms. They are considered as a single ion called a polyatomic ion. You will not need to memorize these, only recognize it when you see them Examples Aluminum chromate Potassium hydroxide Sodium thiosulfate Calcium acetate