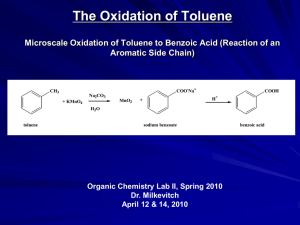
Benzene global demand
advertisement

13/14 Spring Semester Petrochemical Technology (TKK-2130) Instructor: Rama Oktavian Email: rama.oktavian86@gmail.com Office Hr.: M.13-15, Tu. 13-15, W. 13-15, Th. 13-15, F. 09-11 Aromatic compound Aromatic compound Benzene a cyclical, six carbon, six hydrogen molecule a clear, colourless, volatile liquid with a characteristic ‘aromatic’ smell Aromatic compound Benzene http://www.epa.gov/otaq/regs/toxics/airtox1b.pdf Aromatic compound Benzene uses combined and processed with other basic chemicals (such as ethylene or propylene) to produce countless consumer goods The largest derivative outlet for benzene is ethylbenzene production of styrene polystyrene widely used to produce cumene Phenol, cyclohexane, and aniline http://www.shell.com/global/products-services/solutions-for-businesses/chemicals/products/aromatics/benzene.html Aromatic compound Benzene uses Aromatic compound Benzene global demand http://mcgroup.co.uk/researches/benzene Aromatic compound Benzene uses http://www.shell.com/global/products-services/solutions-for-businesses/chemicals/products/aromatics/benzene.html Aromatic compound Benzene uses http://mcgroup.co.uk/uc/2013/05/Global-Styrene-Consumption-by-End-Use-Sector-in-2013.jpg Aromatic compound Benzene global demand According to a new IHS Chemical (NYSE: IHS) global market study, global demand for benzene, an aromatic hydrocarbon and one of the primary chemical building blocks for the petrochemical industry, increased to 43.7 million metric tons in 2013, an increase of 2.8 percent above demand for benzene in 2012. http://press.ihs.com/press-release/aromatic-chemicals/global-demand-benzene-primary-chemical-building-block-chemical-indu Aromatic compound Benzene demands http://www.shell.com/global/products-services/solutions-for-businesses/chemicals/products/aromatics/benzene.html Aromatic compound Benzene sources http://www.chemsystems.com/images/PERP0607_6_BenzeneToluene/0607_6_Fig1.JPG Aromatic compound Benzene sources http://www.2b1stconsulting.com/wp-content/uploads/2012/07/Aromatics-definition.jpg Aromatic compound Benzene production Extractive distillation Catalytic reforming Toluene hydrodealkylation and disproportionation Pyrolysis gasoline Production from coal tar Benzene production Extractive distillation http://www.2b1stconsulting.com/wp-content/uploads/2012/07/Aromatics-definition.jpg Benzene production Extractive distillation Sulfolane Extractive Distillation Technology (SED) http://english.sinopec.com/products_service/License/Petrochemical/20090908/7573.shtml Benzene production Catalytic reforming Reforming takes straight chain hydrocarbons in the C6 to C8 range from the gasoline or naphtha fractions and rearranges them into compounds containing benzene rings A typical catalyst is a mixture of platinum and aluminium oxide Benzene production Production from pyrolysis gasoline Pyrolysis gasoline is the by-product of steam cracking of paraffin gases, naphtha, gas oils and other hydrocarbons used to make ethylene. It contains 60% aromatics, 50% of which is benzene http://www.dow.com/hydrocarbons/aromatics/company/production.htm Aromatic compound Toluene = methylbenzene http://www.sigmaaldrich.com/chemistry/solvents/toluene-center/physical-properties.html Aromatic compound Toluene uses Aromatic compound Toluene uses Aromatic compound TDI uses As raw material for flexible polyurethane (PU) foam used in furniture, mattresses and car seats. rigid foams and adhesives, paints, concrete sealers, as a cross-linking agent for nylon 6, and as an intermediate in PU coatings and elastomers http://www.icis.com/resources/news/2008/01/21/9093901/chemical-profile-tdi/ Toluene Toluene consumption http://mcgroup.co.uk/uc/2013/05/Toluene-2014-World-Market-Outlook-and-Forecast-up-to-2018.jpg Toluene Toluene consumption http://mcgroup.co.uk/uc/2013/05/Toluene-Diisocyanate-TDI-2014-World-Market-Outlook-and-Forecast-up-to-2018.jpg Toluene Toluene production technology Toluene Toluene production technology Toluene Toluene to benzene production technology Toluene hydrodealkylation The hydrodealkylation is conducted either purely thermally at 550-800°C and 30100 bar or catalytically at somewhat lower temperatures of 500-650 °C and 30-50 bar Over Cr2O3, Mo2O3, or CoO on supports (e.g., Al2O3) or, as in a recent development, at 400-480°C over Rh/Al2O3. Toluene Toluene to benzene production technology Toluene disproportionation 2 toluene molecules are reacted and the methyl groups rearranged from one toluene molecule to the other, yielding one benzene molecule and one xylene molecule Toluene Toluene to TDI production process Toluene diisocyanate (TDI) is currently produced via a three-step process: - Nitration of toluene to dinitrotoluene (DNT) Toluene is nitrated in two steps, producing the three isomers of mononitrotoluene. and the mixed mononitrobenzene isomers are further nitrated resulting in dinitrotoluene isomers - Reduction of dinitrotoluene to toluene diamine (TDA) Dinitrotoluene is dissolved in methanol and reduced continuously by reaction with hydrogen in the presence of a suitable catalyst http://www.chemsystems.com/about/cs/news/items/PERP%200708S9_TDI.cfm Toluene Toluene to TDI production process Toluene diisocyanate (TDI) is currently produced via a three-step process: - Phosgenation of TDA to TDI The 80/20 TDA produced by any of the above processes is converted to diisocyanate by reaction with phosgene phosgene http://www.chemsystems.com/about/cs/news/items/PERP%200708S9_TDI.cfm Aromatic compound Xylene the o- isomer has the IUPAC name of 1,2-dimethylbenzene the m- isomer has the IUPAC name of 1,3-dimethylbenzene the p- isomer has the IUPAC name of 1,4-dimethylbenzene Aromatic compound Xylene Properties The melting point ranges from −47.87 °C (−54.17 °F) (m-xylene) to 13.26 °C (55.87 °F) (p-xylene) The boiling point for each isomer is around 140 °C (284.00 °F) The density of each is around 0.87 g/mL (7.26 lb/U.S. gallon) Xylene in air can be smelled at 0.08 to 3.7 parts of xylene per million parts of air (ppm) https://www.princeton.edu/~achaney/tmve/wiki100k/docs/Xylene.html Aromatic compound Xylene uses Aromatic compound Recent update Developing technologies 1. China Petroleum and Chemical Corporation (CPCC) and Sinopec have developed a new composite solvent for extractive distillation (ED) of aromatics. 2. ExxonMobil proposes a bound zeolite catalyst for use in alkylation, transalkylation or isomerization of aromatic hydrocarbons. 3. UOP has developed a new family of zeolites that can be used in alkylation of aromatics, transalkylation of aromatics, isomerization of aromatics and alkylation of isoparaffins. Aromatic compound Recent update Catalyst development in aromatic hydrocarbon production using catalytic pyrolysis 1. Aromatic hydrocarbons production from ex situ catalysis of pyrolysis vapor over Zinc modified ZSM-5 in a packed-bed catalysis coupled with microwave pyrolysis reactor, published in Fuel Volume 129, 1 August 2014, Pages 78–85 Aromatic compound Recent update Catalyst development in aromatic hydrocarbon production using catalytic pyrolysis from biomass 1. Production of aromatic hydrocarbons by catalytic pyrolysis of microalgae with zeolites: Catalyst screening in a pyroprobe, published in Bioresource Technology Volume 139, July 2013, Pages 397–401 Aromatic compound Recent update Catalyst development in aromatic hydrocarbon production using catalytic pyrolysis from biomass 2. Production of aromatic hydrocarbons through catalytic pyrolysis of 5Hydroxymethylfurfural from biomass, published in Bioresource Technology Volume 147, November 2013, Pages 37–42 Aromatic compound Recent update Catalyst development in aromatic hydrocarbon production using catalytic pyrolysis from biomass 3. Production of Light Aromatic Hydrocarbons from Biomass by Catalytic Pyrolysis, published in Chinese Journal of Catalysis Volume Volume 29, Issue 9, September 2008, Pages 907–912