CHE Ch 12 - Lawndale High School
advertisement

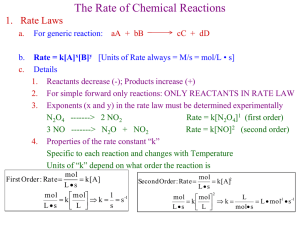
Mr. Samaniego Lawndale High How many moles of ammonia are produced when 0.60 mol of nitrogen reacts with hydrogen? mol = ? (NH3) 0.60 mol N2 0.60 mol N2 + 3 H2 2 _ mol _ NH 3 N2 1 _ mol _ N 2 2 NH3 = 1.2 mol NH3 Balanced equations are necessary to find the amount of product or reactant needed in a chemical reaction. Cu + O2 CuO Calculate the number of grams of NH3 produced by the reaction of 5.40 g of hydrogen with an excess of nitrogen. The balanced equation is mass = ? (NH3) 5.40 g H2 5.40 g N2 + 3 H2 2 NH3 1 _ mol _ H 2 _ mol _ NH 17 . 04 _ g _ NH 2 3 3 H2 2 .02 _ g _ H 2 3 _ mol _ H 2 1 _ mol _ NH 3 = 30.4 g NH3 Lithium nitride reacts with water to form ammonia and aqueous lithium hydroxide. Li3N + 3H2O NH3 + 3LiOH a. What mass of water is needed to react with 32.9 g of Li3N? b. When the above reaction takes place, how many molecules of NH3 are produced? c. Calculate the number of grams of Li3N that must be added to an excess of water to produce 15.0 L of NH3 (at STP). Pages 358-361: 6-14 (skip 11a). (12/14/11, 12/15/11) A + B C grams of A to grams of C: grams A mol A mol C grams C mol of A to grams of C: mol A mol C grams C mol of A to mol of C: mol A mol C grams of A to mol of C: grams A mol A mol C Calculate the number of grams of H2O produced by the reaction of 12.5 g of nitrogen monoxide with an excess of ammonia. The balanced equation is 4NH3 + 6NO 5N2 + 6H2O mass = ? (H2O) 12.5 g NH3 12.5 g 1 _ mol _ NH 6 _ mol _ H O 18 .02 _ g _ H 2 O 3 2 NH3 1 _ mol _ H O 2 17 .04 _ g _ NH 3 4 _ mol _ NH 3 = 19.8 g H2O After Ch 12 Quiz How many molecules of oxygen are produced when 29.2 g of water is decomposed by electrolysis according to this balanced equation? 2H2 + O2 molecules = ? (O ) 2H2O 29.2 g H2O 29.2 g 2 1 _ mol _ H 2 O H2O 18 . 0 _ g _ H 2 O 1 _ mol _ O 2 2 _ mol _ H O 2 6 . 02 x10 _ molecules 1 _ mol _ O 2 23 = 4.88x1023 molecules O2 _ O2 Nitrogen monoxide and oxygen gas combine to form the brown gas nitrogen dioxide, which contributes to photochemical smog. How many liters of nitrogen dioxide are produced when 34 L of oxygen reacts with an excess of nitrogen monoxide? (Assume @ STP.) vol O2 = 34 L vol = ? (NO2) 34 L 1 _ mol _ O 2 O2 22 . 4 _ L _ O 2 Remember: 1 mol = 22.4 L @STP 2NO + O2 2 _ mol _ NO 2 1 _ mol _ O 2 2NO2 22 . 4 _ L _ NO 2 1 _ mol _ NO 2 = 68 L NO2 Assuming STP, how many milliliters of oxygen are needed to produce 20.4 mL SO3 according to this balanced equation? vol SO3 = 20.4 mL vol = ? (mL O2) 20.4 mL SO3 2SO2 + O2 1 _ mL _ O 2 2 _ mL _ SO 3 2SO3 = 10.2 mL O2 Remember: 1 mol = 22.4 L @STP