unit 6 alcohols
advertisement

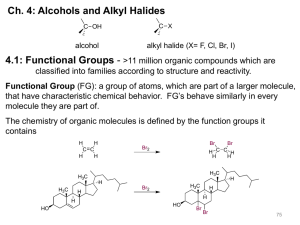
Unit 6 Alcohols and Ethers Alcohols Nomenclature Physical Properties Synthesis Reactions Alcohols contain at least one C-OH bond. are similar in structure to water (an alkyl group replaces one of the H’s in water). The C bonded to the -OH is called the carbinol C atom. Nomenclature of Alcohols methyl alcohol 2° alcohol phenol 1° alcohol 3° alcohol Nomenclature of Alcohols Apply the same rules you learned for the alkanes. Use the root name of the longest chain containing the hydroxyl group, but change -e to -ol. IUPAC: methanol common: methyl alcohol IUPAC: butan-1-ol 1-butanol common: n-butyl alcohol Nomenclature of Alcohols Number the longest C chain containing the -OH, starting at the end nearer the -OH, and use the appropriate number to indicate the position of the -OH. Br OH IUPAC: 6-bromoheptan-3-ol 6-bromo-3-heptanol Nomenclature of Alcohols With a cyclic alcohol, the -OH is assumed to be on the #1 C. CH3 IUPAC: 2-methylcyclohexanol OH Nomenclature of Alcohols Priority: The alcohol is the highest priority functional group of the ones we have studied so far: Alcohols Amines Alkenes/Alkynes Alkanes Ethers Halides Nomenclature of Alcohols OH IUPAC: (E)-hept-5-en-3-ol trans-5-hepten-3-ol OH IUPAC: cyclohex-2-en-1-ol Nomenclature of Alcohols An alcohol group that is a substituent is called “hydroxy”. O OH OH IUPAC: 2-hydroxybutanoic acid Nomenclature of Alcohols Alcohols with two -OH groups are diols. Vicinal diols are called glycols. IUPAC: propane-1,2-diol common: propylene glycol OH OH IUPAC: 4-cyclopentylheptane-3,5-diol. Note the “e.” Nomenclature of Thiols A thiol is an organic compound with an -SH group, the sulfur analog of an alcohol. aka a mercaptan IUPAC: 4-cyclopentylheptane-3-thiol SH Nomenclature of Phenols IUPAC: 2-bromophenol common: ortho-bromophenol IUPAC: 3-bromophenol common: meta-bromophenol IUPAC: 4-bromophenol common: para-bromophenol Nomenclature of Phenols IUPAC: benzene-1,4-diol common: hydroquinone IUPAC: benzene-1,3-diol common: resorcinol IUPAC: benzene-1,2-diol common: catechol Nomenclature of Alcohols Give IUPAC acceptable names. OH 3-(iodomethyl)-2-isopropylpentan-1-ol HO CH2I OH Cl CH2CH2OH (1R,3S)-3-(2-hydroxyethyl)cyclopentanol (Z)-4-chlorobut-3-en-2-ol IR spectrum of alcohol with hydrogen bonding Cyclohexanol, neat SDBSWeb : http://riodb01.ibase.aist.go.jp/sdbs/ (National Institute of Advanced Industrial Science and Technology, 10/16/11) IR spectrum of alcohol with very little hydrogen bonding Cyclohexanol in CCl4 SDBSWeb : http://riodb01.ibase.aist.go.jp/sdbs/ (National Institute of Advanced Industrial Science and Technology, 9/3/11) Physical Properties of Alcohols The -OH group is polar and forms a hydrogen bond. The boiling points of alcohols are high: Most of the common alcohols up to C11 or C12 are liquids at room temperature. The short-chain alcohols are miscible with water. Physical Properties of Alcohols BP increases with the amount of H-bonding. 1-propanol 1,2-propanediol bp = 97°C bp = 188°C propylene glycol 1,2,3-propanetriol bp = 290° glycerol Physical Properties of Alcohols The alkyl part of the alcohol is nonpolar. As the carbon chain gets longer, alcohols become more suitable for dissolving nonpolar compounds. As the carbon chain gets longer, alcohols become less soluble in water. Rule of thumb: One -OH can carry 4 carbon atoms into solution. Physical Properties of Alcohols Like water, the -OH group in an alcohol can act as an acid and lose H+. Higher Ka, more acidic. Lower pKa, more acidic. Physical Properties of Alcohols pKa cyclohexanol 18.0 water 15.7 methanol 15.5 phenol 10.0 acetic acid 4.8 Physical Properties of Alcohols As the previous slide shows, alcohols are weak acids. Only phenols react with NaOH to lose the H+. Other alcohols require a much stronger base before they lose their acid H+. Physical Properties of Alcohols Acidity increases with the ability to pull electrons away from C-O-. e- withdrawing groups stabilize anions (edonating groups stabilize carbocations) pKa=14.3 pKa=12.2 pKa=15.9 pKa=10.0 Physical Properties of Alcohols Phenols are the most acidic alcohols because resonance stabilizes the conjugate bases. …can you draw the resonancestabilized forms of the phenoxide ion? Synthesis of Alcohols - Review from SN2 of -OH with alkyl halides from alkenes acid-catalyzed hydration oxymercuration-demercuration hydroboration-oxidation hydroxylation (1,2-diols) from addition of acetylides to carbonyl compounds Synthesis of Alcohols - New industrial preparation of methanol and ethanol from addition of organometallic reagents to carbonyl compounds from reduction of carbonyl compounds Synthesis of Alcohols - Review from SN2 of -OH with alkyl halides (e.g., NaOH in acetone) inversion of configuration Synthesis of Alcohols - Review from alkenes acid-catalyzed hydration Markovnikov product Synthesis of Alcohols - Review from alkenes oxymercuration-demercuration Markovnikov product anti addition Synthesis of Alcohols - Review from alkenes hydroboration-oxidation syn addition Synthesis of Alcohols - Review from alkenes - syn hydroxylation to make vicinal diols cold, dilute KMnO4 in base or OsO4/H2O2 Synthesis of Alcohols - Review from alkenes - anti hydroxylation to make vicinal diols step 1: make the epoxide CH3CO3H (goes straight to diol if water is present) MCPBA step 2: acidify Synthesis of Alcohols - Review from addition of acetylides to carbonyl compounds Industrial Synthesis of Methanol widely used solvent and fuel Prepared from synthesis gas Δ 3C(coal) + 4H2O CO2 + 2CO + 4H2 Preparation requires a temperature of 300-400°C, H2 pressure of 200-300 atm, and a CuO-ZnO/Al2O3 catalyst: CO(g) + 2H2 (g) CH3OH Industrial Synthesis of Ethanol Solvent and fuel - The pure form is subject to expensive taxes. Denatured alcohol contains impurities that render it undrinkable. Prepared from ethylene: H2C=CH2(g) + H2O CH3CH2OH Preparation requires a temperature of 300°C, an H2O pressure of 100-300 atm, and a catalyst (phosphoric acid adsorbed onto diatomaceous earth). Synthesis of Alcohols - New from addition of organometallic reagents to carbonyl compounds or epoxides formaldehyde aldehydes ketones esters and acid chlorides epoxides In every case, the C chain is lengthened. Organometallic Compounds contain a covalent bond between C and a metal atom. Grignard reagents (R-MgX) organolithium reagents (R-Li) contain a nucleophilic C. Grignard reagents act like R:- +MgX organolithium reagents act like R:-Li+ Organometallic Compounds contain a nucleophilic C. We’ve already encountered a nucleophilic C…in the acetylides. Sodium amide can deprotonate a terminal alkyne, but not an alkene or an alkane. To make an alkane or an alkene act as a nucleophile, convert it into an organometallic compound. Organometallic Compounds Grignard reagents are made from an alkyl halide and Mg in ether. Ether is needed to dissolve the Grignard reagent. CH3I + Mg diethyl ether CH3MgI methylmagnesium iodide Careful: Water destroys the Grignard reagent! Organometallic Compounds Organolithium reagents are made from an alkyl halide and Li in an ether or an alkane. CH3CH2Br + 2Li ether or alkane CH3CH2Li + LiBr ethyllithium Careful: Water destroys the organolithium reagent! Organometallic Compounds CH3CH2Li + H2O CH3CH3 + LiOH ethyllithium Careful: Water destroys the organolithium reagent! Synthesis of Alcohols - New from addition of organometallic reagents to formaldehyde Step 1: Why can’t step 2 be combined with step 1? Step 2: Synthesis of Alcohols - New from addition of organometallic reagents to aldehydes Synthesis of Alcohols - New from addition of organometallic reagents to ketones Synthesis of Alcohols - New from addition of organometallic reagents to esters and acid chlorides to make 3° alcohols with two identical groups. Two (2) moles of the reagent are needed. Synthesis of Alcohols - New Two (2) moles of the reagent are needed. Why? Acid chlorides: Cl- is a good LG, and so, with one mole of the Grignard, a ketone forms. The ketone can be attacked by a second mole of the Grignard reagent. Esters: Now the LG is RO-, not usually considered “good,” but the reaction takes place by nucleophilic acyl substitution, not by SN2. In this mechanism, RO- leaving is exothermic and therefore favorable. Synthesis of Alcohols - New from addition of organometallic reagents to epoxides Grignard reagents will NOT react with ethers, which is why ether is the solvent. The reagent attacks the less hindered (less substituted) C of the epoxide. Side Reactions of Organometallic Reagents Grignard reagents and organolithium compounds are strong bases and strong nucleophiles. They react immediately and irreversibly with water… and any other compound that can protonate a strong base. O-H, N-H, S-H, -C≡C-H Grignard reagents cannot contain the following unprotected groups, as they will be attacked: C=O, C=N, C≡N, N=O. Side Reactions of Organometallic Reagents Water is a poison to these reagents: CH3CH2MgBr + H-O-H CH3CH3(g) + BrMgOH HOCH2CH2CH2CH2Br + 2Li do NOT produce HOCH2CH2CH2CH2Li. The H from the –OH reacts immediately: HOCH2CH2CH2CH2Br + 2Li Li+ -OCH2CH2CH2CH3 + LiBr Side Reactions of Organometallic Reagents BrCH2CH2CH2CHO + Mg does not make a usable Grignard reagent because would react with the C=O of a second molecule of the starting compound. Side Reactions of Organometallic Reagents Grignard reagents can couple with the alkyl halide. Although this does not happen to a great extent, it is an unwanted side reaction that limits the yield of the Grignard reagent. CH3CH2CH2Br + Mg ether CH3CH2CH2MgBr + CH3CH2CH2Br CH3CH2CH2MgBr ether CH3CH2CH2CH2CH2CH3 Coupling of Alkyl Groups Using a Gilman Reagent Lithium dialkylcuprates (aka Gilman reagents) are used when a coupling with an alkyl halide (or vinyl halide or aryl halide) is desired. Gilman reagent Synthesis of Alcohols - New from reduction of carbonyl compounds NaBH4 reduces aldehydes and ketones but not acids and esters. Alcohol, ether, or water is the solvent. LiAlH4 followed by H+ reduces any C=O, including acids and esters. Raney nickel saturated with H2(g) reduces C=O but also C=C. finely divided Ni “honeycomb” with large surface area: fixed bed or slurry NaBH4 and LiAlH4 NaBH4 and LiAlH4 act as Hnucleophiles. In actuality, they deliver H- while limiting its basicity. LiAlH4 (aka LAH) reacts explosively with water and alcohols. LiAlH4 + 4H2O LiOH + 4H2 + Al(OH)3 NaBH4 reacts slowly with water and alcohols if the pH is kept high. NaBH4 + 4H2O NaOH + 4H2 + B(OH)3 Mechanism of Hydride Reduction of Carbonyls Mechanism of Hydride Reduction of Carbonyls H2O may be included with the NaBH4. LAH Reduction of Carbonyls When using LiAlH4, H2O may not be included with it but must be added afterward (in a second reaction step). Because Al is less electronegative than B, LiAlH4 is a stronger nucleophile and will react with less reactive carbonyls such as esters, as well as with the more active aldehydes and ketones. LiAlH4 followed by H+ will separate an ester into two alcohols. Reduction of Carbonyls Thiols (Mercaptans) -SH instead of -OH Thiols are more acidic than alcohols characteristic, unpleasant odor oxidize to give sulfonic acids pKa = 7.8 Predict the Product Cl 1. Mg(s)/ether 2. CH3CO2CH3 3. H3O+ Cl 1. Mg(s)/ether 2. CH3CH2CO2H 3. H3O+ Conversion ? OH D Predict the Product O NaBH4 CH3CH2OH O O 1. LAH 2. H3O+ Predict the Product HO 1. NaH/THF 2. CH3I 1. BH3.THF 2. H O /OH2 2