Stoichiometry Review: Mole Ratios, Limiting Reagents
advertisement

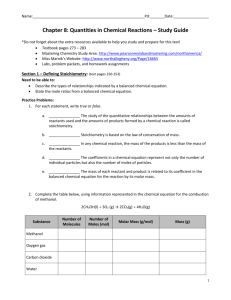
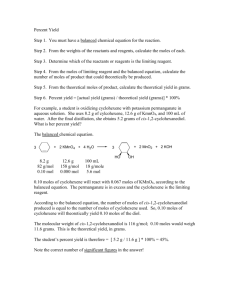
SCH 3U – Unit 3 – Calculations in Chemistry Review Note – Part 2 7.1 Mole Ratio • • A mole ratio converts moles of one compound in a balanced chemical equation into moles of another compound. Example: Reaction between magnesium and oxygen to form magnesium oxide. 2Mg(s) + O2(g) 2MgO(s) Mole ratios: 2 : 1 : 2 Steps: o Write the balanced chemical equation, listing the given values and the required values. o Convert the amount of given substance to the amount of required substance using your mole ratio in the balanced reaction o Statement Try These What amount (mol) of hydrogen is produced when 5.2 mol of ammonia decomposes? Phosphorous, P4, burns in oxygen to produce diphosphorous pentoxide. What amount of oxygen (mol) is required to produce 0.25mol of product? A can of butane lighter fluid contains 1.20 mol butane (C4H10). Calculate the number of moles of carbon dioxide given off when this butane is burned. 7.2 Mass Relationships in Stoichiometry We can use mole ratios numbers to predict the number of moles of product produced We can calculate moles using the mass and molar mass of a compound: n=m/M We can calculate mass using the number of moles and molar mass of a compound: m=nM YOU CANNOT DO STOICHIOMETRY WITHOUT CONVERTING TO MOLES! Steps: o o o o o Write the balanced chemical equation Convert mass to moles using mole equation Use the mole ratio to predict moles of other substance Convert moles to mass using mole equation Statement Mass of A Moles of A Moles of B Mass of B Try These A typical astronaut exhales 8.83 x 102 g of carbon dioxide daily. Determine the mass of lithium hydroxide required to react with this mass of CO2. CO2(g) + 2LiOH(aq) Li2CO3(aq) + H2O(l) An automobile airbag is inflated with nitrogen produced from the decomposition of sodium azide, NaN3. The mass of nitrogen in a fully inflated airbag is 87.5 g. What mass of sodium azide is required to produce this mass of nitrogen? A typical antacid tablet contains 0.50 g of calcium carbonate. What mass of hydrochloric acid will this mass of calcium carbonate neutralize? 7.3-7.4 Limiting and Excess Reagents • • Limiting Reagent: The reactant that is completely consumed in a chemical reactant – this amount also determined the quantity of product that will be formed Excess Reagent: The reactant that is still present after the reaction is complete Steps 1. 2. 3. 4. Balance chemical equation, include given and required values Determine the limiting reagent Determine the amount of products based on limiting reagent Statement Try These • Determine the amount of titanium metal produced when 2.8 mol of titanium(IV) chloride reacts with 5.4 mol of magnesium. • Methanol, CH3OH, can be made using a synthesis reaction involving carbon monoxide and hydrogen. What mass of methanol can be produced from 9.80 g of carbon monoxide and 1.30 g of hydrogen? • Suppose that a solution containing 3.50 g of Na3PO4 is mixed with a solution containing 6.40 g of Ba(NO3)2. How many g of Ba3(PO4)2 can be formed? 7.5 Percentage Yield • • • Theoretical Yield: The maximum amount of product that can be formed in a reaction – determined using stoichiometry. Actual Yield: The amount of product that is actually formed/isolated from a chemical reaction – this is a measured value. Percent Yield: The amount of product that was recovered relative to the theoretical (calculated) amount. Percent Yield = Actual Yield x 100 Theoretical Yield Try These • What is the theoretical yield if 5.50g of hydrogen react with nitrogen to form ammonia? • If 28.6214g of ammonia is produced, what is my percent yield? • 5.3 mol of calcium and 3.8 mol of aluminum chloride are added to the reaction flask. • Are these quantities in stoichiometric amounts? If not determine the limiting reagent. • What is the theoretical yield of aluminum in grams? • 90.521g of aluminum was isolated. What is the percent yield?