Chemical History
advertisement

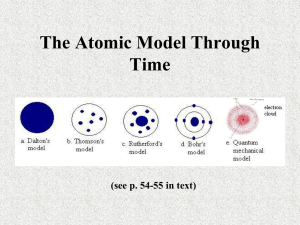
A Quick History of Chemistry With thanks to Isaac Asimov As easy as LMN No one knows where the Latin word “elementum” comes from. We get our word ELEMENT from it. Some think maybe the Romans had an expression that something was as simple as “L-M-N,” just as we say something is as easy as “A-B-C.” We use the word element to refer to a substance which cannot be broken down into a simpler substance. The Ancient Greeks Aristotle suggested that everything was composed of 4 elements: Water, Air, Earth and Fire He later added a 5th element which he called “aether” which the stars and the heavens were made from. Aristotle also said that “cold” was a substance he called primum frigidum, and that “cold” came from water. People actually believed this up until the middle ages (1500s at least). Click on the next slide to see three of these “ancient elements.” Earth Wind and Fire (http://www.earthwindandfire.com/bio.html) Yes, I’m “old school.” But I love these guys. And, I even saw them in concert once, back in the 80’s. OK, back to chemistry history…on the NEXT slide…If you don’t have these guys on your iPod, get some. Tonight. Seriously. Democritus—400 BC (http://www.gap-system.org/~history/Mathematicians/Democritus.html) Democritus was the first to suggest that matter was composed of atoms, which he called “atamos” meaning “indivisible.” Unfortunately, he came from a small “hick town” and people didn’t believe him. Aristotle, for example, ridiculed him. Because Aristotle was more respected, Democritus’ ideas faded into history for over 2,000 years. The Alchemists: 1400-1600? (http://www.crystalinks.com/bacon.html) Roger Bacon was an English alchemist who may have been the first European to invent gunpowder. Alchemists actually discovered some chemistry, but mostly they were trying to figure out a way to turn lead into gold. They failed. However, with nuclear reactions, which weren’t discovered until the 20th century, turning lead into gold is now possible, but it costs more to make the gold (that way) than the gold is actually worth. An Alchemist at work… (http://en.wikipedia.org/wiki/Alchemy) Paracelsus was a famous alchemist who may be depicted in this famous painting. He discovered many of the properties of salt in the 1500s. Salt had been known for thousands of years, and in ancient times was so valuable, it was actually used as money. He was probably the first person to realize that elements could come together to form compounds, with different properties from from the elements they are made from. Paracelsus also is credited with discovering zinc. Robert Boyle…the Last of the Alchemists (http://en.wikipedia.org/wiki/Robert_Boyle) A 17th century British nobleman, he met Galileo and was an alchemist, and maybe the first real scientist. Boyle invented a vacuum pump, did many experiments on gases, and is credited with “Boyle’s Law.” P1 x V1 = P2 x V2 This law states that pressure and volume are “inversely proportional” to each other. More on Boyle Boyle started calling himself a “chymist” because the term alchemist had gotten a bad reputation. The spelling was eventually changed to chemist. He questioned the old Greek notions of the elements. He didn’t believe water was an element, and he experimented on water to try and prove that. Even though he didn’t quite prove that, he believed water to be a compound, and the move away from Aristotle’s theory of the elements had begun. He also experimented with cold temperatures, and whether water froze at different rates based on the initial starting temperature. He also scientifically explained some of the properties of ice. Elements By the early 1600s, a few substances were known, but they weren’t known to be elements yet. Gold and silver and copper and lead had been used since the ancient times, but no one knew they were elements. The alchemists actually did discover 4 elements in the middle ages (As, Sb, Bi and Zn). By 1700, about 14 elements were known. By the end of the 1700’s, around 1783, another 11 were known. Chemistry was evolving during this time, but few chemists paid attention to the quantitative aspects of chemistry. They observed, but they didn’t measure. Phlogiston and Priestly (http://en.wikipedia.org/wiki/Joseph_Priestley) Phlogiston was a theory that explained how things burned. Things that burned would release phlogiston to the air. When a substance had used up all of its phlogiston, it would stop burning. Although it was disproven before he died, he always believed he was right. He also was the inventor of something much more interesting: carbonated beverages. Antione Lavoisier (http://www.answers.com/topic/antoine-lavoisier) Father of Modern Chemistry Proved that air was composed of 1/5 oxygen and 4/5 nitrogen Demonstrated experimentally the principle later renamed “The Law of Conservation of Mass.” Proved that hydrogen and oxygen combine to form water, proving at last that water was a compound. Beheaded on 5/2/1794 by guillotine during the French Revolution at age of 50. More on Lavoisier By insisting on careful measurement and thoughtful experimentation, Lavoisier turned chemistry from a series of interesting observations into a real science. He explained the results that others had gotten. They knew what they had done. Lavoisier helped to explain why these things had happened. He studied combustion reactions and discovered the importance of oxygen in both combustion and respiration (disproving phlogiston in the process). More on Lavoisier He also invented the system of naming chemicals that we use today. Prior to Lavoisier, people who discovered things named them whatever they wished. He also published the first modern chemistry text (Traité élémentaire de chimie) thus spreading his knowledge literally around the world. John Dalton (http://www.intute.ac.uk/sciences/blog/wp-content/uploads/2007/09/johndalton.jpg) A Quaker schoolmaster (became a teacher at the age of 12) who studied all sciences, but made his greatest contributions in chemistry. Developed Atomic Theory and Law of Multiple Proportions. Atomic Theory helped to explain many of the observations that scientists were making. Law of Multiple Proportions helped to explain that 2 elements could combine to form more than 1 compound; for example CO and CO2. Dalton’s Atomic Theory 1. All elements are composed of tiny indivisible particles called atoms. 2. Atoms of the same element are identical. The atoms of any one element are different from those of other elements. 3. Atoms of different elements can chemically combine with one another in small whole-number ratios to form compounds. 4. Chemical reactions occur when atoms are separated, joined or rearranged. Atoms of one element cannot be changed into atoms of another element by chemical rxns. Indivisible? Well, Dalton did this work in the early 1800’s. We know now that atoms are composed of protons, neutrons and electrons. Dalton didn’t know about them—they hadn’t been discovered yet! HOWEVER, the atom is “the smallest part of an element that retains the properties of that element.” So an atom of gold is still gold and is different from an atom of carbon. Dalton’s model of the atom is called the “solid sphere” model. Dmitri Mendeleev Mendeleev organized the Periodic Table by atomic mass. He left “holes” in his table for undiscovered elements and challenged the scientific world to “find them!” In the early 20th century, Englishman Henry Moseley reorganized the Periodic Table by putting it in order of atomic number. Element 101 Md (Mendeleevium) is named after him. Moseley has not been so honored yet. JJ Thompson Discovered the Electron in 1897. (http://www.manep.ch/img/photo/challenges/nanotubes/thompson.jpg) Electrons are negatively charged and have almost no mass at all, compared to a proton. Thompson revised Dalton’s model of the atom with one of his own, called the “Plum Pudding Model.” Plum Pudding Model (http://en.wikipedia.org/wiki/Plum_pudding_model) Plum Pudding is a British dessert in which plums are scattered more or less randomly throughout a cake (the pudding). Thompson knew atoms contained electrons, and knew they were negative. He also knew that the atoms overall were neutral. So, he proposed that the negative electrons were randomly distributed throughout. The rest of the atom was positively charged. Thompson proposed the electrons were moving in a circular fashion within the positively charged “rest of the atom.” Robert Millikan and the Oil Drop Experiment http://educar.sc.usp.br/licenciatura/2003/mi/Millikan-Oil-Drop-Apparatus.gif This is what he used to do it… Robert Millikan measured the exact charge of the electron in “The Oil Drop Experiment.” Atoms can GAIN or LOSE electrons to form ions. Ions are atoms with a charge! Electrons are negatively charged. Each electron has a charge of -1. (Don’t forget the negative sign…it’s VERY important!) Ernest Rutherford’s Nuclear Model…it’s now 1910 or so… (https://reich-chemistry.wikispaces.com/file/view/Ernest_Rutherford.JPG) The Plum Pudding Model wouldn’t last long, because one of JJ’s former students did some experiments that forced the model to be revised again. Like his mentor, JJ Thompson, Rutherford won the Nobel Prize for his work His “work” was the famous “gold foil” experiments, where he was researching alpha particles (see Chapter 28 stuff again). As sometimes happened, Rutherford didn’t set out to discover what he actually did. Rutherford is also credited with discovering the proton around 1919. The Gold Foil Experiment (http://www.mhhe.com/physsci/chemistry/essentialchemistry/flash/ruther14.swf) Check out the link! (http://www.rsc.org/chemsoc/timeline//pages/1911.html) Reference for below… Rutherford created a device to “shoot” α particles at a thin piece of gold foil, literally only a few atoms thick. He expected them to go through with little or no deflection. But that’s NOT what happened. Some bounced straight back as if they had hit a brick wall! Shocked, SHOCKED! Rutherford was completely surprised by this result. He had accidentally discovered the nucleus. Rutherford figured out that most of the mass of the atom was contained in a small, dense center which was positively charged. The electrons still rotated around the nucleus, but most of the atom was composed of “empty space.” Neils Bohr: The Planetary Model & Energy Levels (http://www.usd.edu/phys/courses/phys300/gallery/clark/bohr.html) Rutherford’s nuclear model only really lasted for about 3 years, before Neils Bohr revised it again. Soccer goalie on Denmark’s 1908 Olympic team AND a Nobel Prize winner in 1922!! Talk about student/athlete!” His son also won a Nobel Prize in 1975! Bohr asked a question: if the electrons are rotating around the nucleus, why don’t they “run out of energy.” As they did, they would come closer and closer, attracted by the opposite charge of the nucleus, and eventually collapse onto the nucleus, destroying the atom in the process. This doesn’t happen, and Bohr answered why. Bohr’s Planetary Model (http://www.rsc.org/chemsoc/timeline//pages/1913.html) An old Bohr? But the electrons don’t just orbit anywhere. They can only move in orbits that Bohr called “energy levels.” Each energy level has a certain amount of energy. Electrons can move to a higher energy level by gaining energy. Or they can drop to a lower energy level by losing (or emitting) energy. But they can’t “run out” of energy, because in order to stay in an energy level, they must have that certain amount of energy. Energy Levels http://library.thinkquest.org/C006669/media/Chem/img/bohr.gif An energy level is a “region around the nucleus where an electron is likely to be moving.” The first energy level (n = 1) has the lowest energy. It is called “the ground state.” Things in nature prefer to be in the lowest possible energy state. Spectral Lines for H http://www.daviddarling.info/images/hydrogen_spectrum.gif Electrons can ABSORB energy and move to a higher energy level. This is called “an excited state.” If an electron moves from n=1 (ground state) to n=3, it is in an excited state. When an electron loses energy, it drops to a lower energy level. When an electron loses energy, we The lines are characteristic for hydrogen. say it EMITS energy. If that They are like a fingerprint to identify H. energy is in the visible part The Ballmer series is the only ones you of the spectrum, we can see can see, but the others can be detected. those transitions. More Bohr http://www.daviddarling.info/images/hydrogen_spectrum.gif In Bohr’s model, the energy levels get closer together as you get further away from the nucleus. If the electron gets far enough away from the nucleus, it can escape (n = ∞). The electrons can jump from one level to another. They can jump more than one level at a time by absorbing or emitting enough energy. An electron cannot jump to a spot midway between levels (n ≠ 2.5) We no longer have an atom. We have an ion, since the atom has lost an electron. Need for a Better Model Bohr’s model has some limitations. It worked very well for hydrogen (the simplest atom with only 1 electron). It allowed scientists to make detailed calculations that explains the behavior of H. It didn’t work for other elements, mostly because the calculations were so detailed and complex they couldn’t be done (the math hasn’t been invented yet). It also violated the Heisenberg Uncertainty Principle. But no one knew that yet! We’ll get to that. Heisenberg Uncertainty Principle http://www.ostheimer.at/mambo/images/stories/Werner_Heisenberg_Tafel.jpg Since momentum = mass x velocity and since the mass of the electron is known, for all practical purposes, the Heisenberg Uncertainty Principle says that you can’t know both the position of the electron and the speed of the electron, at the same time. The Heisenberg Uncertainty Principle states that for a very small particle, such as an electron, you cannot know both its exact momentum and its exact position at the same time. So why does Bohr’s model violate Heisenberg’s Principle The Modern Model of the Atom Many scientists (Louis DeBroglie, Max Planck, Albert Einstein, Erwin Schroedinger, and many others) worked on the model of the atom. Actually, they weren’t working on the model of the atom. They were just working on cool and interesting scientific problems. But they all made contributions to our current understanding of the atom. Quantum mechanics is the “modern” model of the atom. By the early 1930s, it had been “born.” It’s the model we still use today. Gee, bet this guy never amounted to anything Photoelectric Effect http://www.guidetothecosmos.com/images/slide12_plus.jpg The photoelectric effect was discovered by Albert Einstein. He found that light of a certain energy could “knock electrons loose” from certain metals. Oh BTW, Einstein published “Theory of Relativity” 6 years later. Wait! Light Knocks Electrons Off of Atoms, if it has Enough Energy? Alkali metals seem to be very prone to this, if the light is of a sufficient energy. Einstein called this the photoelectric effect. In this way, light is behaving not as a wave but as a particle. Photoelectric Effect, So What? Anyway, you might not be terribly impressed with Einstein’s discovery. However, if electrons can be pried loose from the metal, they can move around. If they can move around, the movement of electrons can generate a small amount of electricity. If you can capture this electricity, you can do useful work. Solar Power Solar power is based off of this principle. A photoelectric cell is constructed which has a certain type of metal in it. When sunlight shines on it, some of the electrons are pried loose. The cell generates an amount of electricity. With hundreds or thousands of these in series, you can take a small amount of power generated in each cell, and multiply that by the total number of cells, and use that generated power to do work in your house. OK, well so what? This was one of the assumptions that helped lead scientists to quantum mechanics. While in graduate school in France, a young scientist named Louis de Broglie asked himself this question If light can act as a particle, can a moving particle also act as a wave? De Broglie Equation http://jkphysics.in/images/De-broglie.jpg The answer was yes. λ=h/mxv λ = wavelength h = Planck’s constant m = mass v = velocity The wavelength for a baseball pitched at 90 miles per hour, calculated using de Broglie’s equation is 8.2 x 10-38 meter. We have no measuring instrument capable of detecting such an incredibly small distance. The Final Pieces of the Puzzle But, electrons have masses which are much, much less, and they have wavelengths which can be measured much more easily. So if particles could act as a wave, and electrons are particles, would it help our understanding of the atom to think of electrons as “waves?” The answer was yes and quantum mechanics was the result. Previously, scientists had treated electrons just as particles, and tried to use all the normal math techniques that they used on particles they could see. Those techniques worked well with large particles, but with electrons, not so much. Quantum Mechanics http://www.hmi.de/bereiche/SF/SF7/PANS/english/nobel/Schroedinger/Schroedinger_01.jpg When Erwin Schroedinger recalculated everything using the “wave math” everything started to come together and make total sense. He called this quantum mechanics. Later in life, he actually said this about quantum mechanics… “I do not like it and I regret having had anything to do with it.” To which I add, ditto! His simple-looking but really complex math equation. Still the best model we have! Quantum mechanics has been around for 80 years now. It still predicts the behavior of atoms well, and we haven’t found anything better. If anyone ever finds anything better, I’ll let you know Had Dinner with this Dude http://sunsite.berkeley.edu/CalHistory/photos-large/seaborg.big.jpg Dr. Glenn T. Seaborg That was 2,400 years of history in one lesson! Discovered Plutonium in 1940; won Nobel Prize in 1951; predicted the existence of the actinide series; had dinner with Mr. Schwartz in 1981; element 106 named in his honor in 1997 (Sg); died 1999. There’s lots more to know and explore. Yes, you need to know the important people and what they did. It could be on the SOL! The End Next: Chapter 5 powerpoint…