Solution Chemistry
advertisement

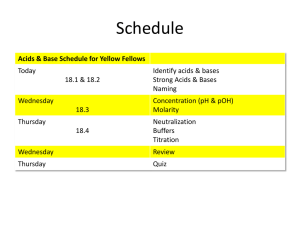
Solution Chemistry Solutions, Problems, Solutions, Problems. Does it ever end? How can you express concentration? How can you express concentration? 1) 2) 3) 4) 5) Mass % composition Molarity Molality Mole fraction Density Mass % problems: • Find mass % • Find amount of solute • Find total mass of solution Mass % problems: • Find mass % • Mass % = mass of solute x 100 % total mass of solution Don’t forget to add the masses of the solute and solvent for the total mass Mass % problems: • Find amount of solute • Mass % = mass of solute x 100 % total mass of solution • Multiply by total mass and divide by 100% to solve for mass of solute Mass % problems: • Find total mass of solution • Mass % = mass of solute x 100 % total mass of solution • Multiply both sides by the mass of solution first to get it out of the denominator. Molarity problems: • Find molarity • Find amount of solute • Find volume of solution Molarity problems: • Find molarity • M= moles solute Volume of solution (L) Molarity problems: • Find amount of solute • M= moles solute Volume of solution (L) • Molarity x volume= moles! Molarity problems: • Find volume of solution • M= moles solute Volume of solution (L) • Multiply both sides by the volume of solution first to get it out of the denominator. OR! Molarity problems: • Find volume of solution • Use the molarity as a conversion factor! • moles solute x 1 L = Volume of solution! M Dilution Problems M x V = Moles • Moles of solute in new solution = moles in the stock solution. Molarity goes down, volume goes up Dilution Problems Example: What is the concentration of a solution made by diluting 25 ml of a .50 M HCl solution to a new volume of 150 ml? Dilution Problems Example: What is the concentration of a solution made by diluting 25 ml of a .50 M HCl solution to a new volume of 150 ml? M x V = moles. .50 M x .025 L= .0125 moles HCl M = mol/vol= .0125 mol / .150 L = .083 M Dilution Problems 1) What is the concentration of a solution made by diluting 125 ml of a 2.5 M NH3 solution to a new volume of 350 ml? 2) What is the concentration of a solution made by diluting 2.5 ml of a 6.0 M NaCl solution to a new volume of 80. ml? 3) What is the concentration of a solution made by diluting 30. ml of a 1.0 M Fe(NO3)3 solution with 20. ml of water? (The final volume should be 20. + 30. = 50. ml) Dilution Problems Example: What volume of a 2.0 M NaOH stock solution is required to mix 1.50 L of a .150 M NaOH solution? Dilution Problems Example: What volume of a 2.0 M NaOH stock solution is required to mix 1.50 L of a .150 M NaOH solution? M x V = moles. .150 M x 1.50 L = .225 moles NaOH .225 moles NaOH x 1 L/ 2.0 moles= .113 L Dilution Problems 1) What volume of a 6.0 M NaOH stock solution is required to mix 1.50 L of a .150 M NaOH solution? 2) What volume of a 2.0 M MgCl2 stock solution is required to mix 1.750 L of a .10 M MgCl2 solution? 3) What volume of a 1.0 M HCl stock solution is required to mix 100. ml of a .10 M HCl solution? Volume Stoichiometry • If you mix 10. ml of .10 M HCl with .10 M NaOH, • …it should take 10. ml of the NaOH to react completely. Volume Stoichiometry • If you mix 10. ml of .10 M HCl with .20 M NaOH, • …it should take only 5.0 ml of the NaOH to react completely. Volume Problems 1) If you react 10.0 ml of .10 M HCl with .050 M NaOH, what volume of NaOH solution will be needed? 2) If you react 10.0 ml of .10 M H2SO4 with .050 M NaOH, what volume of NaOH solution will be needed? 3) If you react 50.0 ml of 1.0 M CaCl2 with 1.9 M Na2CO3, what volume of Na2CO3 solution will be needed? Volume Stoichiometry • If 10. ml of .10 M HCl reacts completely with 10. ml NaOH, • …the concentration of the NaOH must be the same, .10 M Volume Stoichiometry • If 10. ml of .10 M HCl reacts completely with 20. ml NaOH, • …the concentration of the NaOH must be half of that, .050 M. Volume problems • If 25.0 ml of .30 M HCl reacts completely with 25. ml NaOH, what is the concentration of the NaOH? • If 10.0 ml of .10 M HCl reacts completely with 20. ml NaOH, what is the concentration of the NaOH? • If 15.0 ml of .10 M HCl reacts completely with 50. ml Pb(NO3)2, what is the concentration of the Pb(NO3)2? Acids and Bases Examples? • Acids • Bases Properties of Acids and Bases • Acids • Bases Properties of Acids and Bases • Acids Are electrolyte solutions • Make ions in solution • Affect indicators • Have low pH • Taste sour • Neutralize bases Can cause serious burns Corrode reactive metals Have more H+ than OH(in solution) • Bases Are electrolyte solutions • Make ions in solution • Affect indicators • Have high pH • Taste bitter • Neutralize acids Can cause serious burns Corrode aluminum only Have more OH- than H+ (in solution) Properties of Both • Acids Are electrolyte solutions • Make ions in solution • Affect indicators • Have low pH • Taste sour • Neutralize bases Can cause serious burns Corrode reactive metals Have more H+ than OH(in solution) • Bases Are electrolyte solutions • Make ions in solution • Affect indicators • Have high pH • Taste bitter • Neutralize acids Can cause serious burns Corrode aluminum only Have more OH- than H+ (in solution) BrØnsted-Lowry Definition • Substances that donate a proton (H+ ion) in a reaction are acids. • Substances that accept a proton (H+ ion) are bases. Arrhenius acids and bases make H+ and OH- ions in solution. BrØnsted-Lowry bases are also Arrhenius bases. pH • The basic (and acidic) definitions are: pH= -log [H+] [H+]= 10-pH pOH= -log [OH-] [OH-]=10 -pOH Kw=[H+][OH-]=1 x 10 -14 (at 25oC) pH + pOH = 14 (at 25oC) pH practice • If pH is 3.38…. 1) What is the pOH? pH practice • If pH is 3.38…. 1) What is the pOH? 14-pH= 10.62 pH practice • If pH is 3.38…. 1) What is the pOH? 14-pH= 10.62 2) What is [H+]? pH practice • If pH is 3.38…. 1) What is the pOH? 14-pH= 10.62 2) What is [H+]? 10-3.38= 4.17 x 10-4M pH practice • If pH is 3.38…. 1) What is the pOH? 14-pH= 10.62 2) What is [H+]? 10-3.38= 4.17 x 10-4M 3) What is [OH-]? pH practice • If pH is 3.38…. 1) What is the pOH? 14-pH= 10.62 2) What is [H+]? 10-3.38= 4.17 x 10-4M 3) What is [OH-]? 10-10.62=2.40x10-11M and Kw/4.17x10-4M=2.40x10-11 M! pH practice • If [OH-]= 4.8 x 10-6 M… pH practice • If [OH-]= 4.8 x 10-6 M… 1) What is pOH? pH practice • If [OH-]= 4.8 x 10-6 M… 1) What is pOH? -log (4.8 x 10-6 )= 5.32 pH practice • If [OH-]= 4.8 x 10-6 M… 1) What is pOH? -log (4.8 x 10-6 )= 5.32 2) What is pH? pH practice • If [OH-]= 4.8 x 10-6 M… 1) What is pOH? -log (4.8 x 10-6 )= 5.32 2) What is pH? 14-5.32= 8.68 pH practice • If [OH-]= 4.8 x 10-6 M… 1) What is pOH? -log (4.8 x 10-6 )= 5.32 2) What is pH? 14-5.32= 8.68 3) What is [H+]? pH practice • If [OH-]= 4.8 x 10-6 M… 1) What is pOH? -log (4.8 x 10-6 )= 5.32 2) What is pH? 14-5.32= 8.68 3) What is [H+]? 10-8.68= 2.08 x 10-9 M and Kw/ 4.8 x 10-6 = 2.08 x 10-9 M ! Please recall: • Strong acids and bases dissociate completely in water. Weak acids/bases do not. • Strong acids= nitric, hydrochloric, sulfuric, hydrobromic, hydroiodic, perchloric • Strong bases-Group 1 & 2 hydroxides—(group 2’s don’t dissolve much) Please recall: 1. What is the concentration (M) of NaOH if .35 mole NaOH is dissolved in .120 L solution? 2. What is the molarity of HCl if 12 g HCl is dissolved in .85 L of solution? 3. What is [OH-] if .35 g Ba(OH)2 is dissolved in .250 L solution? 4. What mass of H2SO4 is in 55 ml of .38 M H2SO4? Please recall: 1. What is the concentration (M) of NaOH if .35 mole NaOH is dissolved in .120 L solution? 2. What is the molarity of HCl if 12 g HCl is dissolved in .85 L of solution? 3. What is [OH-] if .35 g Ba(OH)2 is dissolved in .250 L solution? 4. What mass of H2SO4 is in 55 ml of .38 M H2SO4? Did you notice? Analyze these solutions Contents pH [H+] (M) [OH-] (M) pOH 1 .023 mol HCl /L 2 1.5g NaOH /L 3 ? mol LiOH / L 4 ? KOH/L 8.50 mol 5 .? gHClO4 /L 6 ? mol Ba(OH)2/L 2.50 .020 .0070 Acidic or Basic Right! pH [H+] (M) [OH-] (M) 1 .023 mol HCl /L 1.64 .023 4.3x10-13 12.36 Acidic 2 1.5g NaOH /L 12.57 2.7x10-13 .0375 1.43 Basic 3 3.2 x10-6 mol LiOH / L 8.50 3.2x10-9 3.2x10-6 5.50 basic 4 3.2 x10-3 mol KOH/L 11.50 3.2x10-12 3.2x10-3 2.50 basic 5 2.0 gHClO4 /L 1.70 6 .0035 mol Ba(OH)2/L 11.85 1.4x10-12 .0070 Contents .020 pOH Acidic or Basic 5.0x10-13 12.30 acidic 2.15 basic Conjugates • After an acid has donated a proton, the rest of the species is the conjugate base. HAA- + H+ • After a base has accepted a proton, the resulting species is the conjugate acid. B- + H+ HB What is the conjugate base of… • • • • • • • HCl CH3COOH H2SO4 HSO4H2O NH4+ NH3 What is the conjugate base of… ACID (loses H+ to form its) Conjugate base • HCl • CH3COOH • H2SO4 • HSO4• H2O • NH4+ • NH3 What is the conjugate base of… ACID (loses H+ to form its) Conjugate base • HCl ( H+ and) Cl• CH3COOH • H2SO4 • HSO4• H2O • NH4+ • NH3 What is the conjugate base of… ACID (loses H+ to form its) Conjugate base • HCl ( H+ and) Cl• CH3COOH( H+ and) CH3COO• H2SO4 ( H+ and) HSO4• HSO4( H+ and) SO4-2 • H2O ( H+ and) OH• NH4+ ( H+ and) NH3 • NH3 ( H+ and) NH2- What is the conjugate acid of… • • • • • • NO3C2O4-2 HPO4-2 HSO4H2O F- What is the conjugate acid of… Base (gains H+ to form its) Conjugate acid • NO3• C2O4-2 • HPO4-2 • HSO4• H2O • F- What is the conjugate acid of… Base (gains H+ to form its) Conjugate acid • NO3(+H+ ) HNO3 • C2O4-2 • HPO4-2 • HSO4• H2O • F- What is the conjugate acid of… Base (gains H+ to form its) Conjugate acid • NO3(+H+ ) HNO3 • C2O4-2 (+H+ ) HC2O4• HPO4-2 (+H+ ) H2PO4• HSO4(+H+ ) H2SO4 • H2O (+H+ ) H3O+ • F(+H+ ) HF Nomenclature • If the anion name • ends in…. then the acid name is… Fill in the blanks • • • • • HCl is _____________acid HClO4 is _____________acid HClO3 is _____________acid HClO2 is _____________acid HClO is _____________acid Hydrogen chloride Hydrogen chlorate Fill in the blanks • • • • • Hydrogen perchlorate HCl is _____________acid HClO4 is _____________acid HClO3 is _____________acid HClO2 is _____________acid HClO is _____________acid Hydrogen chlorite Hydrogen hypochlorite Nomenclature • • • • • • • If the anion name ends in…. --ide (hypo--) --ite --ite --ate (per--) –ate then the acid name is… Hydro___ic acid Hypo___ous acid ___ous acid ___ic acid Per ___ic acid Fill in the blanks • HNO3 is _____________acid • HIO4 is _____________acid • H2CO3 is _____________acid • H3PO3 is _____________acid • HBrO is _____________acid Fill in the blanks • _____________is hydrocyanic acid • _____________ is perbromic acid • _____________ is phosphoric acid • _____________ is sulfurous acid • _____________ is hypoiodous acid Show the conjugate acid/base pairs in the following reactions. • HC2O4- + HNO3 H2C2O4 + NO3- • HC2H3O2 + PO4-3 HPO4-2 + C2H3O2 - Show the conjugate acid/base pairs in the following reactions. Acid • HC2O4- + HNO3 Base • HC2H3O2 + PO4-3 Conjugate Base H2C2O4 + NO3Conjugate Acid HPO4-2 + C2H3O2 - Show the conjugate acid/base pairs in the following reactions. Acid • HC2O4- + HNO3 H2C2O4 + NO3Conjugate Acid Base Base • HC2H3O2 + PO4-3 Acid Conjugate Base Conjugate Acid HPO4-2 + C2H3O2 Conjugate Base [H+] is inversely related to [OH-] • When [H+] increases, [OH-] decreases in a water solution, and vice versa. Review question: 125 ml of a KOH solution is mixed so that the pH is 12.23 1) What is the pOH, [OH-] and [H+]? 2) What is the [KOH] ? 3) How many moles KOH was used? 4) What mass of KOH was used? (FMKOH= 56.1 g/mol) Review question: 125 ml of a KOH solution is mixed so that the pH is 12.23 1)pOH=1.77;[OH-]=.0170M;[H+]=5.88x10-13M 2) [KOH]=[OH-]= .0170M (it’s a strong base!) 3)moles=MxV=.0170Mx.125L=.00213mol 4) massKOH =molesKOHx FMKOH = .00213mol x 56.1 g/mol =.119 g Strength of acids and bases. • HCl • H2CO3 Strength of acids and bases. • HCl -- strong acid • H2CO3 -- weak acid Strength is determined by amount of dissociation Strength of acids and bases. • HCl -- strong acid, it dissociates completely • H2CO3 -- weak acid, dissociates partly • What about their conjugates? Strength of acids and bases. • Cl• HCO3- Strength of acids and bases. • Cl- -- not a base • HCO3- -- weak base Strength is determined by amount of association Strength of acids and bases. • Cl- -- not a base, it does not associate with water. • HCO3- -- weak base, it associates partly • What about their conjugates? Strength of acids and bases. The conjugate of a strong acid is not a base The conjugate of a weak acid is a weak base The conjugate of a strong base is not an acid Strength of acids and bases. The conjugate of a strong acid is not a base The conjugate of a weak acid is a weak base the stronger the acid, the weaker the base and vice versa The conjugate of a strong base is not an acid When dissolving acids and bases… • For an acid, HA • For a base, B- When dissolving acids and bases… • For an acid, HA HA (aq)H+ (aq) +A- (aq) • For a base, BB- (aq) +H2O (l) HB (aq) +OH- (aq) Write the reaction for: • Ammonia associating with water • Ammonium dissociating in water Write the reaction for: • Ammonia associating with water NH3(aq) + H2O (l) NH4+ (aq) + OH- (aq) • Ammonium dissociating in water NH4+ (aq) H+ (aq) + NH3 (aq) Write the reaction for: • Cyanide associating with water • Oxalic acid dissociating in water On your test, you will be asked to: • • • • • • • • • Calculate molarity in dissolving, dilution, & titration Calculate mass % composition Calculate mass and moles of solute in solutions Calculate volume of solution Calculate pH, pOH, [H+], and [OH-] Name acids and write formulas for conjugates Pick out acids, bases, and conjugates in a reaction Write association and dissociation reactions Compare strength of acids and bases
![pH = - log [H + ]](http://s2.studylib.net/store/data/005622524_1-002df1ea50d2a849b15deb604928664e-300x300.png)