Acids and Bases
advertisement
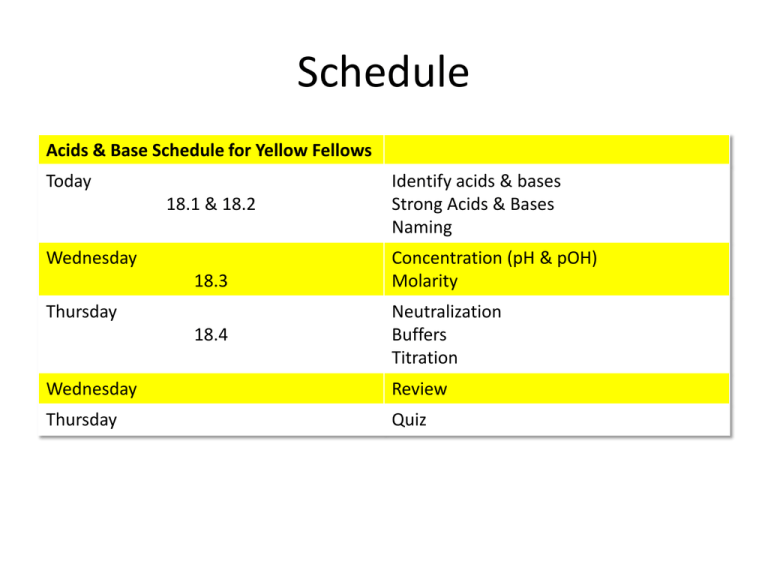
Schedule Acids & Base Schedule for Yellow Fellows Today 18.1 & 18.2 Wednesday 18.3 Thursday 18.4 Identify acids & bases Strong Acids & Bases Naming Concentration (pH & pOH) Molarity Neutralization Buffers Titration Wednesday Review Thursday Quiz Schedule Acids & Bases Schedule Team Tree Today 18.1 & 18.2 Wednesday 18.3 Double Lab 18.4 Identify acids & bases Strong Acids & Bases Naming Concentration (pH & pOH) Molarity Neutralization Buffers Titration Wednesday Review Friday Quiz Unilever’s Bread & Butter The Chemistry of Love… • Initial attraction through nonverbal communication is often attributed to phermones. • “Raw lust” is characterized by high levels of testosterone? • The sweaty palms and pounding heart of infatuation are caused by higher than normal levels of norepinepherine. • The 'high' of being in love is due to a rush of phenylethylamine and dopamine. • Lasting love confers chemical benefits in the form of stabilized production of serotonin and oxytocin. • Can infidelity be blamed on chemistry? Perhaps in part. Researchers have found that suppression of vasopressin can cause males (voles, anyway) to abandon their love nest and seek new mates. A delicious acid Schedule Acids & Bases Schedule for Lava Lovers Tuesday (2/14) 18.1 & 18.2 Wednesday (2/15) Identify acids & bases Strong Acids & Bases Naming 18.3 Concentration (pH & pOH) Molarity 18.4 Neutralization Buffers Wednesday (2/22) Friday Double Lab (2/23) 18.4 Titration Lab Quiz Today’s Objective – 18.1 & 18.2 • To identify acids and bases using the Arrhenius, Brønsted-Lowry and Lewis models. • To identify strong & weak acids and bases. • To name acids & bases. • Tonight’s homework: – read section 18.3 & take notes – mini quiz tomorrow Content: What is an Acid? • Arrhenius model: A species that contains hydrogen and produces hydrogen ions in an aqueous (water) solution. HX H+ + X– Real example: HCl H+ + Cl- Content: What is an Acid? • Brønsted-Lowry model: hydrogen ion donor. Produce conjugate acids and bases HX(aq) + H2O H3O+(aq) + X-(aq) –Real example: HF(aq) + H2O H3O+(aq) + F-(aq) Content: conjugate acids and bases acid base conjugate acid conjugate base • conjugate acid: the species produced when a base accepts a hydrogen ion. • conjugate base: the species that results when an acid donates its hydrogen ion. Content: What is a base? • Arrhenius model: species that produces hydroxide ions in an aqueous solution. MOH M+ + OH– Real examples: NaOH Na+ + OH- Content: What is a base? • Bronsted-Lowry model: hydrogen ion acceptor. NH3(aq) + H2O(l) NH4+(aq) + OH-(aq) base acid conjugate acid conjugate base Content: Water – both acid & base H 2 O + H2 O • Known as: amphoteric • Complete the equations. In each, is water an acid or base? – HX + H2O – NH3 + H2O Summarize: Compare & Contrast 3 words or less Model Arrhenius Brønsted-Lowry Acid Definition Base Definition Summarize: Make Connections • What real world substances are classified as acids and bases? • What ion is likely present in acids? Today’s Objective – 18.2 & 18.3 • To classify strong acids and bases v. weak acids & bases. • To explain pH and pOH. • Tonight’s homework: – worksheet if you don’t finish it in class. Content: Strong Acids and Bases • Strong: completely ionizes in aqueous solutions – Strong acid equation • HX (aq) – Strong base equation • MOH Strong Acids & Bases Memorize Acids • HCl • HBr • HI • HNO3 • H2SO4 • HClO4 Bases • Hydroxides of group 1 or 2 metals – Ex: NaOH, KOH, Mg(OH)2 Content: Weak acids & bases • Only partially dissociate in water. Reaction does not go to completion, is reversible. • Use double arrow – Weak acid reaction • HB (aq) – Weak base reaction • NH3(aq) + H2O What does this look like? • http://phet.colorado.edu/en/simulation/acidbase-solutions Check for Understanding • Classify the following as weak/strong acid/base. – HF – LiOH – HBr – NaOH – NH3 Check for Understanding • What would happen to the following substances in water? Write an equation for each and draw what this would look like – HF -LiOH – NaOH -NH3 – HBr Classification of acids and Bases • If [H+] > [OH-] = • If [OH-] > [H+] = • If [H+] = [OH-] = Today’s Objective • To understand pH. • To neutralize an acid using titration. 18.3 Ion Product Constant for Water • Pure water contains equal concentrations of H+ and OH– ions. • The ion production of water Kw = [H+][OH–] = 1.0x10-14 • a number that doesn’t change, a constant number. • equals the concentration of the H+ and OH– ions. Ion Product Constant for Water • In an acidic or basic solution, as the [H+] goes up, [OH-] goes down, and vice versa so Kw remains the same. The pH scale • Scientific notation is cumbersome, so it’s re-expressed using logs. • pH is the negative logarithm of the hydrogen ion concentration of a solution. pH = –log [H+] Did she just say “log?!” • 103 = 1000 o 10 is the base. o 3 is the exponent. • A logarithm is basically solving for exponents, x in the following o 10x=1000 o here x = 3 • log101000 = x • log101000 = 3 • "The log of 1000, base 10, is 3” logbase(number) = exponent So for pH… • If [H+] = 1.0 x 10-7 • and pH = - log [H+] • then pH = - log [1.0 x 10-7] = - (-7) log 10 • and pH = 7 x 1 = 7 • so this would be a neutral solution. The pOH scale • pOH is the negative logarithm of the hydroxide ion concentration of a solution. pH = –log [OH-] pH + pOH = 14 Concentration of Strong Acids & Bases For all strong monoprotic acids (ones that have one H+ ion to donate) the concentration of the acid is the concentration of H+ ions. For all strong bases, the concentration of the OH– ions is the concentration of the base. A 0.6M solution of NaOH means there are 0.6 moles of NaOH per liter of water. NaOH Na+ + OHFor every mole of NaOH, there is one mole of OH-, so the concentration is the same. Neutralization • What happens if we mix a strong acid and a strong base? • A neutralization reaction is a reaction in which an acid and a base in an aqueous solution react to produce a salt and water. • The net reaction (without the salt) is: Titration • It’s all in the technique… Titration • Titration is a method for determining the concentration of a solution by reacting a known volume of that solution with a solution of known concentration. HCl + NaOH NaCl + H2O • If I have 50 mL of a 0.5M solution of HCl, how much of a 0.5M solution of NaOH should be needed to neutralize it? How can we use this to determine the concentration of an unknown acid or base? Titration Procedure • In a titration procedure, a measured volume of an acid or base of unknown concentration is placed in a beaker, and initial pH recorded. Titration Procedure • A buret is filled with the titrating solution of known concentration, called a titrant. Titration Procedure • Measured volumes of the titrant are added slowly and mixed into the solution in the beaker. • The pH is read and recorded after each addition. pH indicator solution • Chemical dyes whose color are affected by acidic and basic solutions are called acid-base indicators. • An end point is the point at which an indicator used in a titration changes color. • An indicator will change color at the equivalence point. End Point • The process continues until the reaction reaches the equivalence point, which is the point at which moles of H+ ions from the acid equals moles of OH– ions from the base. • An abrupt change in pH occurs at the equivalence point. Expected Titration Curve Let’s Practice Naming Bases • Bases – Group 1 and 2 hydroxides… – NH3 (ammonia) Naming Acids • Binary Acids (hydrogen + 1 element) – Prefix “Hydro” to name the hydrogen part of the compound – The rest of the word consists of a form of the root of the second element, plus the suffix “ic” – Second word is always “acid” • • • • Example: HCl Hydrochloric Acid Example: HF Hydrofluoric Acid Example: HI Example: HBr Naming Acids • Oxyacids (contain hydrogen and oxyanion) – Identify oxyanion. The first word is the oxyanion name and a suffix • If original suffix was “ate” “ic” • If original suffix was “ite” “ous” – Second word = “acid” • • • • • • Example: HClO4 = Perchloric Acid Example: HClO3= Chloric Acid Example: HClO2 = Example: HClO= (adds chlorine to pools) Example: HNO3 Example: HNO2 In Conclusion • Using the pH scale, identify what makes a substances acidic, basic or neutral • What is more dangerous, an acid or a base? • What ion is responsible for an acidic solution? A basic solution? • Name the following acids • • • • • H2SO4 H2SO3 HCl HF H2CO3



![pH = - log [H + ]](http://s2.studylib.net/store/data/005622524_1-002df1ea50d2a849b15deb604928664e-300x300.png)