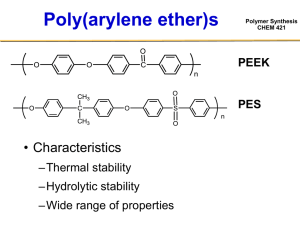
Polymer Synthesis CHEM 421
advertisement

Polymer Synthesis CHEM 421 • Odian Book Chapter 3-15, 5-3 Polymer Synthesis CHEM 421 Living Polymerization (II) by Ru-Ke Bai Department of Polymer Science and Engneering University of Science and Technology of China A Brief Review Polymer Synthesis CHEM 421 • What is living polymerization? • No termination • No chain transfer • What are the major criteria for living polymerization? A B C PDI Mn PDI PDI can be prepared by ln[M]0/[M]t Mn Block copolymers sequential addition of monomers. conversion Time Living polymerization is a good tool for the preparation of block copolymers. Rankings of Anions… Polymer Synthesis CHEM 421 How to characterize the reactivity of a propagating anion? CH H CH + CH3 CH3 C H C C O C + H O OR OR R H R H [H Ka = ][R ] pKa = - log Ka [RH] H Propagating Anions pKa of conjugate acid of prop.chain end CH Styrenes, dienes 42 Acrylic esters 25 CH 3 C C O O R CH 3 C Acrylonitrile 25 C N CH3 C C O R Vinyl ketones 20 Polymer Synthesis CHEM 421 Propagating Anions pKa of conjugate acid of prop.chain end CH2CH 2O Cyclic oxides 16-18 Cyclic siloxanes Si O O Si Si CH3 Si O O CH3 CN Cyano acrylates C C O OCH3 10-12 11-13 Polymer Synthesis CHEM 421 Initiators Useful Initiator Styrenes.dienes 42 RLi, NH2-, Ar - Acrylates 25 RMgX, DPHL Acrylonitriles 25 RO -, C5H5- Vinyl ketones, Aldehydes Cyclic oxides 20 RO - 16-18 RO - Siloxanes(-D3) 10-12 HO -, RO - Cyano acrylates 11-13 H2O, HO -, RO - Super glue Nitroalkenes 10 KHCO3, H2O most nucleophilic pKa Least nucleophilic Reactivity Monomer Polymer Synthesis CHEM 421 Microstructure of Dienes Polymer Synthesis CHEM 421 Four different microstructures in polyisoprene cis 1-4 isomer (natural rubber) trans 1-4 isomer 1-2 isomer 3-4 isomer cis-1,4 favored in hydrocarbon solvents Can MMA be polymerized via living process ? Side reactions 1) 2) 3) Polymer Synthesis CHEM 421 PMMA via Living Pzn Polymer Synthesis CHEM 421 PMMA homopolymer • Can not use BuLi directly • Make new initiator (use 1,1-diphenylethylene) 1,1-diphenyl hexyl lithium Bu n-BuLi Li CH2 C (DPHL) • Sterically hindered • resonant stabilization CH 3 MMA Bu -78 oC, THF CH 2 C CH 2 Li C C O O CH 3 PMMA via Living Pzn Polymer Synthesis CHEM 421 CH 3 CH3 MMA DPHL Bu CH2 C CH2 -78 oC, THF CH2 C C O C O O CH3 O CH 3 CH 3 Methanol Bu CH 2 C CH2 Li C H C -78 oC, THF C O O CH 3 Block Copolymers Polymer Synthesis CHEM 421 • Definition: Macromolecules consisting of homogenous segments made from different monomers (usually two or three different monomers). Ex. A-A-A-A-A-A-B-B-B-B-B-B Some Basic Diblock Copolymer Architectures • Linear Polymer Synthesis CHEM 421 Graft Ex. PS-b-PI Ex: PS-g-PI Star Microphase Separation • Most polymers are immiscible Polymer Synthesis CHEM 421 Block Copolymer Uses Thermoplastic Elastomer Polymer Synthesis CHEM 421 Common Elastomer Poly(cis-1,4-butadiene) SBS (PS-PB-PS) Physical crosslinking Thermal reversibility Can process it repeatedly heating cooling Sulfur Crosslinking Chemical rosslinking Thermal irreversibility Can’t process it repeatedly Self-Assembly of Block CopolymerPolymer Synthesis CHEM 421 Polym. Chem. 2011, 2, 1018–1028. PB-b-PEO cryoTEM micrographs PS-b-PAA TEM micrographs vesicles Cylindrical micelles Spherical micelles = packing parameter, v = hydrophobic volume, area at the hydrophobe-hydrophile/water interface, length normal to the surface per molecule. a = interfacial = the chain Dispersion of Carbon Nanotubes by Polymer Synthesis CHEM 421 Block Copolymer • The study of Single-Walled Carbon Nanotubes (SWNT) composite materials has been hindered by the poor solubility and processibility of SWNTs. • PS-b-PAA has been used to stabilize SWNT and prevent their aggregation. • The micelle-encapsulated SWNTs are compatible with a wide variety of solvent and polymer matrices, which can be used to produce carbon nanotube materials. Kang, Y. and Taton, A. T. J. Am. Chem. Soc. 2003, 125(19) 5650 – 5651. Synthesis of Block Copolymers 1) A-B diblock or A-B-A triblock copolymers same pKa, same reactivity; no problem any order of addition, can cross over back & forth x z y 2) ethylene oxide/styrene copolymers • • • styrene pKa= 42 epoxide pKa= 16-18 cross over from ethylene oxide not possible O H x y Polymer Synthesis CHEM 421 Styrene-MMA Block Copolymers Polymer Synthesis CHEM 421 3) Styrene & MMA • Styrene • Then MMA, but can’t do sequential addition will attack so use 1,1-diphenylethylene C C O OCH 3 sterically hindered, aromatic stablization, won't propagate itself Styrene-MMA Block Copolymers Polymer Synthesis CHEM 421 1) Styrene 2) cap w/ 1,1-diphenylethylene (DPE) 3) MMA @ -78 oC, THF C PS PMMA PMMA-PS-PMMA PMMA Polymer Synthesis CHEM 421 PMMA PS 2 sec-BuLi 55 oC Li Li 1) DFI to initiate styrene 2) Diphenyl ethylene to initiate MMA segment 3) Add MMA DPE DPE DFI PMMA PS PS PMMA Synthesis of Regular Star PS by Iterative Methodology Using DPE Functionality X = Y Polymer Synthesis CHEM 421 = 1st Iteration Br 1 3 O 1st Iteration (= ) 1st Iteration 2st Iteration 3st Iteration 4st Iteration 5st Iteration (= ) 3 O 3 (= 3 ) Synthesis of Asymmetric Star-Branched Polymers by Iterative Methodology Br 3 1 Polymer Synthesis CHEM 421 Synthesis of Asymmetric Star-Branched Polymers by Iterative Methodology Br 3 1 Polymer Synthesis CHEM 421 Synthesis of Star-Branched PS with up to 63 Arms by Iterative Methodology O 3 O 3 Br Polymer Synthesis CHEM 421 5 O 3 O 3 Branched Polymers with Complex Architectures Macromol. Rapid Commun. 2010, 31,1031-1059. star-linear-star (dendrimer)-linear-(dendrimer) graft-on-graft star-on-graft Polymer Synthesis CHEM 421 star-on-linear (dendrimer)-on-linear graft-on-star star-on-star Living/Controlled Free Radical Polymerization Polymer Synthesis CHEM 421 How to perform a living free radical polymerization? Anionic polymerization Radical polymerization kt = 0 kt = 106-108 Ri > Rp R i < Rp Reversible termination Terminology: “controlled/living”, “pseudo-living”, “quasi-living”, and “reversible deactivation radical polymerization” Stable Free-Radical Polymerization (SFRP) Polymer Synthesis CHEM 421 TEMPO: 2,2,6,6-tetramethyl-1-piperidinoxyl • Radical was formed differently • Reversible chain termination! M. K. Georges, et al, Macromolecules, 26, 2987( 1993). Atom Transfer Radical Polymerization Polymer Synthesis CHEM 421 (ATRP) X = Br , Cl Components: Monomer: A wide variety of monomers Initiator: R-X, X = Br and Cl Catalyst: Cu, Fe, and Ru etc. • Radical was formed differently • Reversible chain termination! Ligand: Bipyridine ect. Wang, J. S.; Matyjaszewski, K. Macromolecules 1995, 28, 7901-7910. Reversible Addition-Fragmentation Chain Polymer Synthesis CHEM 421 Transfer (RAFT) • Normal radical initiators (AIBN, etc.) • Reversible chain transfer! Rizzardo, E., et al. Macromolecules 1998, 31, 5559-5562. Advantages of Living Free Radical Polymerization Radical polymerization • A variety of monomers, including the monomers with OH, COOH groups; • Perform in bulk, solution, emulsion, and suspension systems; Polymer Synthesis CHEM 421 Anionic polymerization • Styrenes, dienes, and methacrylates; • Perform in solution under unaerobic and anhydrous conditions; • Complex and expensive. • Simple and inexpensive. A powerful platform for preparing a variety of well-defined polymers