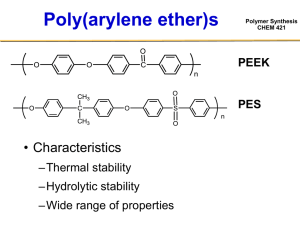
Polymer Synthesis CHEM 421
advertisement

Polymers Used in Microelectronics and MEMs An Introduction to Lithography Page 1 NSF STC Integrated Circuits Polymer Synthesis CHEM 421 Micro-electro-mechanical Devices (MEMS) Polymer Synthesis CHEM 421 Moore’s Law Polymer Synthesis CHEM 421 Year Processor Name Transistor Count Minimum Feature size 1971 1972 1974 1976 1978 1982 1985 1989 1993 1997 1999 (Feb.) 1999 (Oct.) 2000 Source: Intel 4004 8008 8080 8085 8086 80286 80386 Intel486 Pentium Pentium II Pentium III Pentium III Pentium IV 2300 3500 6000 6500 29000 134,000 275,000 1.2 million 3.1 million 7.5 million 9.5 million 28 million 42 million 10 micron 10 micron 6 micron 3 micron 3 micron 1.5 micron 1.5 micron 1 micron 800 nanometer 350 nanometer 250 nanometer 180 nanometer 130 nanometer Industry Road Map Polymer Synthesis CHEM 421 The Drivers in Microelectronics Polymer Synthesis CHEM 421 • Cost: more for less! –$1000 bought: 16MB in 1993 1000MB in 2000 –A single transistor costs about the same as a single printed word in a local newspaper AMD Athlon chip Local Newspaper 22 million transistors 80 pages x 1600 words per page $200 $0.50 J. Phys. Org. Chem. 2000, 13, 767. The Drivers in Microelectronics Polymer Synthesis CHEM 421 • Size – Wafer processing time independent of feature dimension »Printing smaller features or larger wafers allows a greater number of devices to be made in the same amount of time, improving manufacturing yields • Speed –Smaller feature sizes also improve computing speeds by decreasing the travel distance of electrical signals The Chip-making Process Polymer Synthesis CHEM 421 Example – A State-of-the-Art $5 Billion Fab Line Up to 20X 1 Time Semiconductor Manufacturing Polymer Synthesis CHEM 421 Photolithographic Process Polymer Synthesis CHEM 421 • Spin Coat • Expose • Bake • Develop Silicon Substrate • Etch • Strip Process can be repeated up to 30 times: Solvent Intensive! Imaging Process Polymer Synthesis CHEM 421 Handbook of Microlithography, Micromachining and Microfabrication v. 1, P. Rai-Choudhury, ed. SPIE Optical Engineering Press, 1997. Photolithographic Process Photoresist Substrate Polymer Synthesis CHEM 421 Coat h Negative Mask Positive Exposure Develop Etch Strip J. Phys. Org. Chem. 2000, 13, 767. Important Properties of a Photoresist • Resist Thickness (etch resistance) • Solubility for deposition/development • Wettability • Lithographic performance –Sensitivity, contrast • Transparency (more important for 193 nm and beyond) Polymer Synthesis CHEM 421 Optics of Imaging Polymer Synthesis CHEM 421 R = resolution = smallest feature size R / NA • is the wavelength of light • NA is the numerical aperture (a function of the optics) Wavelength Notation Source Feature Size 365 nm i-line mercury lamp 365+ nm 248 nm 193 nm 157 nm DUV 193 nm 157 nm KrF excimer laser ArF excimer laser F2 excimer laser 500 - 100 nm 130 - 70 nm* 90 - 45 nm* Magic!!!!! (aka phase shifting masks…) Synthesis “Transitions” in Optical LithographyPolymer CHEM 421 365 nm G- and I-line Resists Polymer Synthesis CHEM 421 OH • Novolac resin – Base-soluble positive resist (TMAH) – Variety of structures and MW’s OH CH2 CH3 CH3 • Diazonapthaquinone (DNQ) – Photoactive compound (Wolfe Rearrangement) – Inhibits base-dissolution of novolac O O O C CO2H N2 h H2O -N2 R R R R G- and I-line Resists Dissolution Rate (nm/sec) 1,000 — novolac resin & photocatalysis products 100 — novolac resin 10 — 1— novolac resin & DNQ 0.1 — Polymer Synthesis CHEM 421 G- and I-line Resists Polymer Synthesis CHEM 421 • An “engineer’s approach” • Fast N2 outgassing can damage the resist film –Controlled by using a less-intense light source or a less sensitive resist • Wavelength limited resolution (350 nm) • Low contrast (competitive rates of dissolution) Synthesis “Transitions” in Optical LithographyPolymer CHEM 421 365 nm 248 nm Evolution from I- and G-Line to 248 nm (DUV) Polymer Synthesis CHEM 421 • Demand increases for smaller features: R / NA • Diazoquinone novolac photoresists lacked sensitivity at 248 nm • Introduced at 0.365 micron (365 nm) Motivation for Chemical Amplification Polymer Synthesis CHEM 421 • Challenges Encountered: – First exposure tools for 248 nm had low output intensity – Need increased sensitivity to avoid use of extremely bright sources, which are expensive • Chemical amplification invented (Frechet, Willison and Ito) Exposure to photons initiates a chain reaction or promotes a cascade of reactions (500-1000) that changes resist solubility in exposed regions Chemical Amplification Polymer Synthesis CHEM 421 CH CH2 • DUV exposure generates catalytic amount of acid from a photoacid generator (PAG) CH CH2 H+ O O O • 1-2 min PEB to trigger deprotection O + H O O CH CH2 H+ O O • Catalytic chain length is extremely long – About 500 - 1000 carbonate cleavages per proton OH CH H CH2 O C O OH J. Phys. Org. Chem. 2000, 13, 767. Acc. Chem Res. 1994, 27, 150. Photoacid Generators (PAG) 2,6-Dinitrobenzyl tosylate Polymer Synthesis CHEM 421 New fluorinated PAGs Ionic PAG Mechanism Photolysis of diaryliodonium salts Polymer Synthesis CHEM 421 Synthesis 2-Nitrobenzyl Ester PAG MechanismPolymer CHEM 421 o-Nitrobenzyl Rearrangement DUV Resists Polymer Synthesis CHEM 421 Extremely high contrast Initial resistance in manufacturing setting Applicable at i-line with sensitizers Levinson, Harry J. Principles of Lithography. SPIE Press, 2001.