J. J. Thomson
advertisement

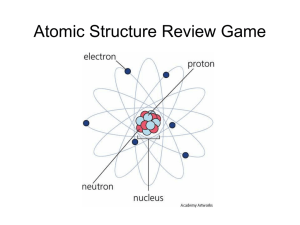
Denae and Rebecca Chemistry 11 Early Life Born: December 18, 1856 Cheetham Hill, Manchester, England Family: Mother – Emma Swindells Father – Joseph James Thomson (ran an antiquarian bookshop) Brother – Frederick Vernon Thomson (two years younger) Education: He went to small private schools where he demonstrated great talent and an interest in science. At only 14 years of age, he was admitted to Owens College in 1870. His parents planned to enroll him as an apprentice engineer to Sharp-Stewart & Co., but these plans changed when his father died in 1873. Later Life Education: In 1876, he moved to Trinity College, Cambridge. In 1880, he got his BA in mathematics (Second Wrangler and 2nd Smith's prize) and MA (with Adams Prize) in 1883. In 1884 he became Cavendish Professor of Physics. One of his students was Ernest Rutherford. Family: Wife – Rose Elisabeth Paget Son – George Paget Thomson Daughter – Joan Paget Thomson Died: August 30, 1940 He was buried in Westminster Abbey, close to Sir Isaac Newton. Idea of the Atom discovered the electron and then created the first model of an atom with the electrons (plum pudding) formed the idea that in a positively charged sphere there was negative electrons that moved/rotated model was later proven wrong by his student, Ernest Rutherford believed that the more energy added, the more the electrons velocity increase, if there was enough energy, they could leave their atoms First Experiment First, he built a cathode ray tube with a metal cylinder on the end. It had two slits in it, leading to electrometers, which could measure small electric charges. He found that by applying a magnetic field across the tube, there was no activity recorded by the electrometers and so the charge had been bent away by the magnet. Therefore, proving that the negative charge and the ray were inseparable and intertwined. Second Experiment Next, he proved that atoms were negatively charged. He did so by modifying the cathode ray tube, originally built by William Crookes, by exposing the cathode ray to an electric field and a magnetic field. The beam deflected toward the positive plate, which meant that there must have been mysterious particles with a negative charge. Third Experiment Then, he figured out that there is a ratio between the mass of the particles and the deflection. He then proved that the negative charged particles where smaller then an hydrogen ions, which was believed to be the smallest atom. Soon after, Thomson used a further-modified cathode ray to show that there were also positively charged particles called protons. Contributions Discoveries: The electron A model of the atom Many isotopes Hydrogen has only one electron per atom A method for separating different atoms and molecules by using positive rays Other: Determined that cathode rays were beams of electrons Determined the mass-to-charge ratio of an electron Proposed the "plum-pudding" model of the atom Argued that the number of electrons in an atom was approximately equal to the atomic weight of that element Worked on the conduction of electricity in gases One of Thomson's greatest contributions to science was in his role as a highly gifted teacher. Seven of his research assistants and his son won Nobel Prizes in physics. Accomplishments 1884: Elected a fellow of the Royal Society 1894: Royal Medal 1902: Hughes Medal 1906: Nobel Prize for Physics in recognition of the great merits of his theoretical and experimental investigations on the conduction of electricity by gases." 1908: Knighted 1910: Elliott Cresson Medal 1912: Appointed to the Order of Merit 1914: Copley Medal 1914: Gave the Romanes Lecture in Oxford on “The atomic theory” 1915-1920: President of the Royal Society 1918: Became Master of Trinity College, Cambridge 1922: Franklin Medal 1937: His son won the Nobel Prize for proving the wavelike properties of electrons 1991: The “thomson” (symbol: Th) was proposed as a unit to measure mass-to-charge ratio in mass spectrometry Bibliography Pictures: http://2.bp.blogspot.com/ALZsMzupHPE/Tbx_I5tU5EI/AAAAAAAAABg/2EHNgL9e2h0/s1600/Nobel_me dal.jpg http://upload.wikimedia.org/wikipedia/commons/thumb/c/c1/J.J_Thomson.jpg /200px-J.J_Thomson.jpg http://cdn.dipity.com/uploads/events/30e9aae2fa011b24e974ab13eb3c11f7_1M.pn g http://web.visionlearning.com/events/images/JJThomson_child.jpg http://electrapk.com/wp-content/uploads/2011/07/hydrogenatom.jpg Information: http://en.wikipedia.org/wiki/J._J._Thomson http://www.nobelprize.org/nobel_prizes/physics/laureates/1906/thomsonbio.html http://www.aip.org/history/electron/jj1897.htm http://www.experiment-resources.com/cathode-ray.html