Chapter 3 Matter, Energy, And Life
advertisement

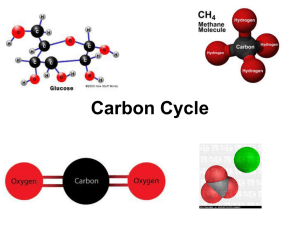
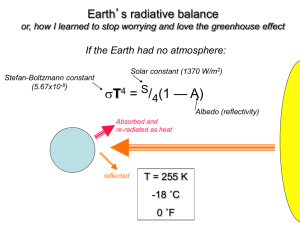
Chapter 3 Matter, Energy, And Life Matter Is Made Of Atoms, Molecules, And Compounds • Atom: simplest building block of chemicals • Element: a material composed of identical atoms • Compound: a combination of atoms in a fixed arrangement and proportion • Molecule: The simplest chemical unit of a compound (O2, H2O, CH4, C6H12O6 etc.) – Many materials (NaCl) don’t have molecules Chemical Formulas • Most Elements have symbols that are common sense: H (Hydrogen), Si (Silicon), etc. • Some, known in ancient times, have symbols from Latin: Fe (Ferrum = Iron), Au (Aurum = Gold), Na (Natrium = lye, for Sodium) • C6H12O6 = Glucose = 6 Carbon, 12 Hydrogen, 6 Oxygen • SiO2 = Quartz = 2 Oxygen for each Silicon Electrical Charge Is An Important Chemical Characteristic • Atoms contain three kinds of particles: – Protons (+) in the nucleus. Number of protons determines what an element is – Neutrons (0) in the nucleus. Bind the nucleus together – Electrons (-) orbiting the nucleus • Group together into shells • This is what interacts with other atoms • Atoms can gain or lose electrons and become electrically charged (Ions) Chemical Bonds Hold Molecules Together • Ionic: Ions of opposite charge attract each other. Example: NaCl, most minerals • Covalent: Atoms share electrons with neighbors. Example: Most carbon chemicals • Metallic: Electrons wander freely between atoms. Positive atoms held together by negative electron “glue” • Hydrogen: H and O in water molecules attracted to neighbors Chemical Bonds Hold Molecules Together • Ionic bonding holds most rocks and minerals together • Covalent bonding holds living things together • Metallic bonding holds industrial civilization together • Hydrogen bonding gives water its solvent and heat-storing capacity Elements Of Life • C, H, O, N, P, S are principal elements of life • Some elements like C can share more than one electron with a neighbor (multiple bonding) • Some elements like Fe and S can gain or lose electrons in more than one way • These versatile atoms can be used for – Energy storage – Information storage – Triggering chemical reactions Elements and Life • Some very abundant elements have no biological uses (Al, Si, Ti) • Some elements are essential in low amounts but toxic at greater levels (Cu, Se) – Everything is toxic at excessive levels • Some elements are toxic and have no biological functions (Lead, Mercury) The Elements The Elements and Life Organic Compounds Have A Carbon Backbone • Organic compounds contain carbon as their basic structural core – Chains (Petroleum) – Rings (Benzene, Toluene) • Simple carbon-bearing chemicals aren’t considered Organic – CH4: Methane – CO2: Carbon Dioxide – CaCO3: Calcite, the Main Constituent of Limestone Cells Are The Fundamental Units Of Life • Cell Membrane: Contains contents and processes, excludes foreign objects (mostly) • Nucleus: Where DNA resides – Simplest organisms lack nucleus • Mitochondria – Not to be confused with Midichlorians (MTFBWY) – Produce Energy for Cell – Have their own DNA – Probably originated as independent organisms Energy • Energy Occurs In Different Types And Qualities • Thermodynamics Regulates Energy Transfers • Energy For Life – Extremophiles Live In Severe Conditions – Green Plants Get Energy From The Sun – Photosynthesis Captures Energy While Respiration Releases That Energy Thermodynamics Regulates Energy Transfers • First Law: Energy is Not Created or Destroyed – Can Change Form – Matter and Energy can be converted • Second Law: Entropy increases – Entropy is often likened to disorder but is not entirely the same – Entropy can decrease at expense of surroundings From Species To Ecosystems • Organisms Occur In Populations, Communities, And Ecosystems • Food Chains, Food Webs, And Trophic Levels Link Species • Ecological Pyramids Describe Trophic Levels Waterworld Sometimes It Looks More Like This Reasons to be a ”Water chauvinist". • Stays liquid over a wide range of temperatures. • Polar or asymmetrical molecule. Attracts ions easily - Good transporter of nutrients • Does not dissolve organic molecules (so we do not dissolve in our own cell fluids) Material Cycles And Life Processes • Sources: supply elements for life and physical processes – Example: Burning vegetation releases CO2 • Sinks: remove materials from environment – Example: Plants remove CO2 from the air – Limestone removes CO2 from the air • Residence Time: How long an average atom or molecule remains in a system – Example: Water molecule in air, 10 days Material Cycles on the Earth • The Hydrologic Cycle Moves Water Around The Earth – Oceans – Atmosphere – Land - Ocean • Nutrient Cycles – Ultimate Source: Rocks – Released by Weathering – Taken up by Biosphere – Transported by Water or Atmosphere – Sinks: Atmosphere, Deep Oceans, Rocks Reasons to be a "Carbon chauvinist". • Can bond to four neighboring atoms • Can bond to other carbon atoms, sharing one, two, or three electrons • These properties make it possible to form a vast array of organic molecules • No other element has these properties Carbon in the Earth • Volcanoes emit carbon dioxide • Carbonate rocks lock up carbon dioxide • Ancient biomass locked up carbon as coal, petroleum, natural gas Carbon in the Biosphere Plants use sunlight, H2O, CO2 to create organic molecules: • 6 H2O + 6 CO2 + energy C6H12O6 (glucose) + 6O2 (toxic waste) Animals run the reactions in reverse: • C6H12O6 (glucose) + 6O2 6 H2O + 6 CO2 + energy • Also use organic molecules directly (vitamins) Carbon Cycles • • • • • Plant – Animal Cycle Decay returns CO2 to atmosphere Marine organisms fix CO2 in carbonate rocks Weathering returns CO2 to atmosphere Some C fixed in rocks long-term as carbonates or fossil fuel • Humans burn fossil fuel and add (not return) CO2 to atmosphere The Carbonate-Silicate Cycle • Earth has almost as much carbon dioxide as Venus • Volcanoes add carbon dioxide to the atmosphere • Mountain-building favors cooling • Carbon dioxide is removed from the air to make carbonate rocks • “Icehouse” and “Greenhouse” episodes The Paradox of Nitrogen • It makes up 79% of the atmosphere • Most plants cannot use N2 • Nitrogen converted to usable forms by specialized microorganisms • Human use of nitrogen – Nitrogen-fixing plants (Legumes) – Natural fertilizers (Guano, Nitrate Minerals) – Synthetic nitrates (Haber Process) Sulfur in the Earth • • • • Sulfide minerals: ores, pyrite Volcanic emissions: H2S, SO2 Coal: pyrite, organic sulfur Petroleum: organic sulfur From Earth to Environment • Volcanic emissions: H2S, SO2 • Microbial action • Weathering – Natural exposures – Mine waste • Smelting • Fossil Fuels Acid Rain • • • • S + O2 = SO2 (sulfur dioxide) 2SO2 + O2 = 2SO3 (sulfur trioxide) SO3 + H2O = H2SO4 (sulfuric acid) Forms by smelting or burning fossil fuels Acid Rain • pH: Measure of acidity – 0 = extremely acid (Muriatic Acid) – 7 = neutral – 14 = extremely alkaline (Lye) • Normal water in air is 5.5 (Carbonic Acid) • Acid rain can be pH 3 or less • Ca and Mg neutralize acid (Limestone, Dolomite, some volcanic rocks) • Rocks poor in Ca and Mg cannot neutralize acid (Granite) Phosphorus in the Earth • Most common limiting factor for life • Mostly in apatite Ca5(Cl,F)(PO4)3 – Granites – Phosphate Rock (recycled biological P) • Released by: – Weathering – Mining (for fertilizer) Phosphorus on Land • • • • Phosphorus in Soil Uptake by plants Consumption by animals Return to soil via plant and animal waste, decay • Some lost by runoff Phosphorus in Water • Essential to aquatic life • Excess causes eutrophication – Runaway productivity, excess oxygen demand • Return to water via plant and animal waste, decay • Some ends up in sediments (Chitin, Bone) • Sedimentary P returns to land via uplift, plate tectonics • Human-Applied P goes to Oceans (Sink) Distinctive Aspects of the P Cycle • • • • • No Atmospheric Component Geologic Portion of Cycle Very Slow Mostly involves biological transfers P in oceans not recycled quickly Human use: Rocks – Fertilizer – Oceans – Not Recycled • Peak Phosphorus? • Phosphorus (Fertilizer) – Morocco, China, South Africa, Jordan, U.S. = 90% of World Reserves