CONGENITAL MALFORMATIONS
AND
TERATOLOGY
TERMINOLOGY
•TERATOGEN = AN AGENT THAT DAMAGES THE SOMATIC
TISSUE OF AN EMBRYO
•MUTAGEN = AN AGENT THAT CHANGES THE GENETIC
MATERIAL OF GERM CELLS
•Most mutagens are teratogens, but few teratogens appear to be
mutagens
•Malformation - morphologic defect resulting from an intrinsically
abnormal developmental process (structure is abnormal from its
inception)
•Deformation - abnormal form, shape, or position of a structure,
caused by mechanical factors
•Disruption - morphologic defect resulting from extrinsic
interference with a normal process
•Dysplasia - abnormal organization of tissue
•Syndrome: "a recognizable pattern of anomalies which are known
or thought to be causally related" (Spranger et al, 1982; Khoury et al,
1994)
TERMINOLOGY
•Sequence: “pattern of anomalies derived from a known (or
presumed) malformation or mechanical factor”
•Complex: "those groups of heterogeneous disorders with
overlapping characteristics that are difficult to separate into specific
conditions" e.g., facio-auriculo-vertebral spectrum, hypoglossiahypodactylia (Martinez-Frias, 1995)
•Association: "derivatives of causally nonspecific disruptive events
acting on developmental fields" or "abnormal markers of normal
embryologic relationships“ (Lubinsky, 1986). In a sense, this is a
"wastebasket" category that should constantly change with
increasing understanding.
•Developmental Field: "basic biological units of individual
development and of evolution, and association to represent the
idiopathic occurrence of multiple congenital anomalies during
blastogenesis" (Opitz, 1985)
CONGENITAL MALFORMATIONS ETIOLOGY
•A. Malformations are present from birth
•B. Causes
–1. Genetic factors: chromosomal (numerical or structural) or gene
abnormalities account for about 15% of malformations
–2. Environmental: a drug, disease, causative agent, malnutrition, etc.
account for about 10% of malformations
–3. About 25% of malformations are due to multifactorial inheritance: a
combination of genetic predisposition and environmental agents
CONGENITAL MALFORMATIONS ETIOLOGY
•C. The presence of malformations is high
in spontaneous abortions, indicating that
the genetic defect is so severe that it halts
embryogenesis; according to Connor and
Ferguson-Smith (1991), chromosomal
findings (those related to VISIBLE
chromosomal defects) in early spontaneous
abortions in the United Kingdom are:
–40% of embryos have apparently normal
karyotopes
–60% of embryos have abnormal karyotypes:
–30% exhibit trisomy
–10% exhibit Turner syndrome (45, XO)
–10% exhibit triploidy, usually due to double
fertilization or to diploid sperm (60% =69,
XXY; 40% = 69, XXX)
–5% exhibit tetraploidy
–5% exhibit various conditions
–Connor and Ferguson-Smith (1991) state that
at least 7.5% of all conceptions have a
chromosomal disorder
Critical Periods in Development
•Cells are changing and
following
specified
developmental
programs
•Interference with one
stage
prevents
the
proper sequence of
events
•There is a limited time
frame in which sets of
cells can complete their
tasks.
Critical Periods in Development
•During this time cells
can be very sensitive
to disruptive agents
•This is the "critical
period" for organ or
tissue
•E.g. disruption of
neural tube closure
leads to spina bifida,
incomplete
palate
formation leads to
cleft palate, etc.
II. GENETIC FACTORS
•A. Genetic Abnormalities may
be signaled by a common
phenotype
–1. Characteristic facial features seen,
for example, in Trisomy 21 (Down's
syndrome); Trisomy 18 & Trisomy
13; Trisomy XXY (Klinefelter
syndrome)
–2. Light colored skin, hair, and eyes
seen in phenylketonuria
•Phenylketonuria is one of the
commonest inherited disorders occurring in approximately 1 in 10,000
babies born in the U. S.
•It occurs in babies who inherit two
mutant genes for the enzyme
phenylalanine hydroxylase (PAH)
II. GENETIC FACTORS
•B. Chromosomal
abnormalities include:
–1. Numerical abnormalities
usually caused by
nondisjunction;
•a. Monosomy = the loss of a
chromosome due to nondisjunction
during oogenesis or
spermatogenesis
•1. Loss of an autosome is lethal
•2. Loss of a sex chromosome
results in Turner syndrome (45,
XO)
TURNER SYNDROME (X Monosomy, Gonadal Dysgenesis)
–a. Turner's syndrome is present
in 1/2500 female births, but 99% of
•Turner embryos spontaneously
abort;
•Turner's syndrome is due to
nondisjunction with the loss of an
entire chromosome or it can be due
to deletion of the short arm of the X
–b. The manifestations include:
short stature, webbed neck, primary
–Amenorrhea (due to ovarian
dysgenesis); congenital heart
malformations
–c. Turner's syndrome is usually due
to the deletion of the paternal X
chromosome
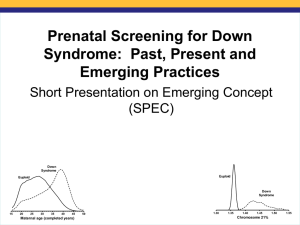
Maternal Age Incidence of trisomy 21 at
birth
20
1/1500
25
1/1350
30
1/900
35
1/380
39
1/150
43
1/50
45
1/28
TRISOMY
•a. Trisomy 21, Down's syndrome
–1. The manifestations of Trisomy 21
include: Mental retardation, simian
crease, epicanthic folds, cardiovascular
and gastrointestinal malformations
–2. Individuals with trisomy 21 can be
expected to present eventually with
presenile dementia; the progressive
deposition of beta amyloid protein in the
cerebral hemispheres is noted at autopsy
in trisomy 21 patients
–3. Virtually all trisomy 21 individuals
who live into their 40's have
–Alzheimer's disease; thus current,
research on Alzheimer's disease in the
general
population
seeks
genetic
dysfunction associated with chromosome
21
–4. The incidence of trisomy 21 increases
with increasing maternal age;
TRISOMY
•b. Trisomy 18,
Edward's syndrome:
1:3000
–The manifestations of
trisomy 18 include:
•
•
•
•
•
mental retardation;
micrognathia;
rocker bottom feet;
deformed ears;
prominent occipital
region;
• only about 10% of these
babies live beyond the
first year
TRISOMY
•c. Trisomy 13, Patau's
syndrome: 1:5000
–The manifestations of
trisomy 13 include:
•
•
•
•
mental retardation;
bilateral cleft lip/palate;
small eyes;
only about 10% of these
babies live beyond the first
year
TRISOMY
•d. XXY:
Kleinfelter's
syndrome; 1:1080
–The individual is
phenotypically male
(because
of
Lyonization),
–tall with long legs,
–a short body, small
testes (he is infertile).
TRISOMY
•e. XXX: Triple X; 1:960
–The individually is
phenotypically female and of
clinically normal appearance;
fertile; but 15-25% are mildly
mentally retarded
•f. XYY:
"Supermale",1:1080
(20/1000 in jails)
–The individual is phenotypically
male with a normal appearance;
tends to be tall;
–personality disorders are fairly
common, especially increased
aggressiveness
STRUCTURAL ABNORMALITIES OF CHROMOSOME
•a.
Translocation:
a
fragment of a chromosome
is moved ("trans-located")
from one chromosome to
another - joins a nonhomologous chromosome.
•The balance of genes is
still normal (nothing has
been gained or lost) but can
alter phenotype as it places
genes
in
a
new
environment.
•Can also cause difficulties
in
egg
or
sperm
development and normal
development of a zygote.
•Acute
Myelogenous
Leukemia is caused by this
translocation
STRUCTURAL ABNORMALITIES OF CHROMOSOME
•b. Deletion: the chromosome
breaks and the small piece
without the centromere is lost
–Cri-du-Chat Syndrome 46 XX
or
XY,
5p(segmental
deletion)
• wailing, cat-like cry in about 50% of
those afflicted
• microcephaly
• severe mental retardation
• heart and other organ deformities
• essentially, this is a partial
monosomy
STRUCTURAL ABNORMALITIES OF CHROMOSOME
•c. Duplication: parts of the
chromosome are duplicated
and are found either within the
same chromosome or on
another chromosome or as an
independent piece
–Fragile X syndrome (MartinBell syndrome)
–Most people have 29 "repeats"
at this end of their Xchromosome, those with Fragile
X have over 700 repeats due to
duplications. Affects 1:1500
males, 1:2500 females.
STRUCTURAL ABNORMALITIES OF CHROMOSOME
•d. Inversion: a portion of
the
chromosome
is
reversed
•e.
Isochromosome:
centromere
divides
transversely instead of
longitudinally
STRUCTURAL ABNORMALITIES OF CHROMOSOME
•C. Mutant genes
–1. Homozygous: both alleles at the locus
are identical (AA, or aa)
–Heterozygous: alleles at the locus are
different (Aa)
–2. Mendelian diseases are due to a single
mutant gene and are inherited acording to
Mendelian laws
–a. Autosomal diseases are encoded on
one of the 22 pairs of autosomes; Xlinked diseases are encoded on the X
chromosome
–b. Dominant genes code for traits that
are expressed in both the homozygote and
the heterozygote (AA, Aa); recessive
genes code for traits that are expressed
only in the homozygote (aa)
Some Genetic Mutations Lead to Abnormal Development
AUTOSOMAL DOMINANT
Condition
Characteristics
Achondroplasia
Dwarfism, mainly due to
shortening of limbs
Neurofibromatosi Multiple neural crest
s
derived tumors on skin,
abnormal pigment areas
on skin
Polycystic
Kidney Disease:
Adult onset Type
III
Numerous Cysts in
Kidneys
AUTOSOMAL RECECIVE
Condition
Characteristics
Albinism
Absence of Pigment
Congenital
Phocomelia
syndrome
Limb Deformities
Polycystic Kidney
Disease: Perinatal
Type I
Numerous Cysts in
Kidneys
X-LINKED
RECESSIVE
Condition
Characteristics
Hemophilia
Defective Blood Clotting
HydrocephalusTesticular
Enlargement of Cranium
Feminization Syndrome
Inability to Respond to
Testosterone Results in
Female Phenotype
Chemical Teratogens Cause Birth Defects in Humans
SOME CHEMICAL TERATOGENS IN HUMANS
Agent
Alcohol
Effect on Human Development
Growth & Mental Retardation, Microcephaly,
Various Malformations of the Face & Trunk
Chemotheurapeutic Agents
(Methotrexate, Aminopterin)
Variety of Major Anomalies throughout the Body
Diethystilbestrol (DES)
Cervical & Uterine Abnormalities
Lithium
Hear Anomalies
Mercury
Mental Retardation, Cerebral Atrophy, Spasticity,
Blindness
Streptomycin
Hearing Loss, Auditory Nerve Damage
Tetracycline
Hypoplasia & Staining of Tooth Enamel, Staining
of Bones
Thalidomide
Limb Defects, Ear Defects, Cardiovascular
Anomalies
Viruses, Bacteria & Protozoa Can Cause Birth Defects
ORGANISMS THAT MAY CAUSE BIRTH DEFECTS
ORGANISM
Rubella Virus
Cytomegalovirus
Trepanoma pallidum
(Spirochete bacterium)
Toxoplasma gondii
(Protozoan)
DISEASE
CONGENITAL DEFECTS
German Measles
Cataracts, Deafness, Cardiovascular
Defects, Slow Growth of Fetus
Cytomegalic Inclusion Disease
Microcephaly, microphthalmia, cerebral
calcification, slowing of intrauterine
growth
Syphilis
Dental Abnormalities, Deafness, Mental
Retardation, Skin & Bone Lesions,
Meningitis
Toxoplasmosis
Microcephaly, Hydrocephaly, Cerebral
Calcification, Mental Retardation
The Mechanism of Action of Teratogens
•a. Inhibition of mitosis
and/or increase in the length
of the cell cycle
•b. Inhibition of protein
synthesis
•c. Inhibition of cellular
migration; known to be
involved in some forms of
mental retardation and in
facial malformations
•d. Damage to DNA, such as
chromosomal
breaks,
nondisjunction and anaphase
lag
The Mechanism of Action of Teratogens
•e. Inhibition of nucleic
acid synthesis
•f. Cell death beyond the
compensatory capacity of
remaining cells
•g. Inhibition of cell
differentiation, including a
slow
down
of
developmental
events
resulting in developmental
delay.
•h. Production of vascular
dysfunction leading to a
disruption of perfusion to
embryonic cells
Agents Affecting Critical Period
•Teratogen: any agent that
disrupts a developing embryo
such as the following:
•Mutated Genes: interfere with
developmental program
•Physical Agents: X-Rays,
Heat
•Chemical Agents: Steroids,
Alcohol, Drugs
•Viruses: herpes, Rubella
(German Measles) viruses
•Other: lack of a specific,
essential component can also
lead to abnormal development
(e.g., FA & Spina Bifida)
Timing of Teratogen Action
Teratogens can have different effects at different stages
Rubella affects different tissues depending on time of infection
Thalidomide at 3 weeks: causes defects in CNS
Thalidomide at 4 weeks: causes defects in the heart
Thalidomide at 3-8 weeks: causes defects in limb development
Fetal Alcohol Syndrome (FAS)
•Babies with FAS typically have a
small head size, a narrow upper
lip, an indistinct philtrim (the pair
of ridges that runs between the
middle of the upper lip and the
nose), and a low nose bridge.
•Abnormalities in neuron migration
and a smaller brain size reflect the
reduced mental ability of FAS
children.
•Abel and Sokal (1987. Drug and
Alcohol Dependence 19: 51-70)
note that While fragile-X and
Down syndrome lead to the most
cases of mental retardation,
•FAS is third, affecting as many as
1 in 500-750 children in the US.
Fetal Alcohol Syndrome (FAS)
•FAS children have a mean IQ of ~68
making them unable to manage their
own lives or to learn from past
experience.
•There are likely many reasons for these
effects of ethanol.
•Neural crest migration is hindered in
the presence of ethanol leading to
premature differentiation of facial
cartilage (Hoffman and Kulyk, 1999.
Int. J. Dev. Biol. 43: 167-174).
•Ethanol also induces apoptosis (death)
of neurons (Ikonomidou et al, 2000.
Science 287: 1056-1060).
•These are just some of the insights that
are being gained as researcher pursue
this serious problem that has high costs
for society and for the affected
individuals.
Cleft Lip & Cleft Palate
•Definition: a facial birth
defect
(congenital
abnormality)
•Common; ~ 1/1000
•Cleft Lip: affects the upper
lip, from a notch to a fissure
into the nose
•Cleft Palate: affects the roof
of the mouth, with a groove
that may extend through the
dental arch (hard palate).
•Abnormalities may occur
separately or together
•Reason: left & right sides do
not contact & fuse
Spina Bifida (SB)
•A neural tube defect
•Caused by the failure of the fetus’s neural
tube to close properly during the first month
of pregnancy
•SB Infants may have an open lesion on their
spine with nerve/spinal cord damage
•SB may also cause bowel and bladder
complications
•Many children with SB have hydrocephalus
(excessive accumulation of cerebrospinal
fluid in the brain).
•Spinal opening can be surgically repaired
nerve damage can’t be fixed, leads to
varying degrees of paralysis of lower limbs
•Three Most Common Forms of SB
•Myelomeningocele: the severest form-spinal cord and its protective covering (the
meninges) protrude from an opening in the
spine
•Meningocele:
spinal
cord
develops
normally but meninges protrude from a
spinal opening
•Occulta: the mildest form, one or more
vertebrae are malformed and covered by a
layer of skin.
Spina Bifida (SB)
• Problems: physical & mobility difficulties, most
individuals have some form of learning disability
• Early detection is done using amniocentesis
• Large amounts of AFG (alpha-fetoprotein) indicates that
Spina Bifida is likely
• Ultrasound reveals a typical morphology after 20 weeks:
Cranial scalloping is evident as a "lemon sign" in the BF
fetus
• Fetus makes a -fetoprotein
• Normal: small amount crosses placenta & enters blood
• Abnormal: high or low levels
• (MSAFP) maternal serum a -FP screening
• Opening in spine (spina bifida) or head, or an abdominal
wall defect: a -FP more leaks out
• Low a -FP levels cause concern, too (Down Syndrome,
Trisomy 18) reasons for low a -FP unknown
• SURGERY: to close the newborn’s spinal opening done
~24 h after birth to preserve existing function in the spinal
cord.
• TREATMENT: There is no cure for SB; Many need
assistance devices such as braces, crutches, or wheelchairs.
• PROGNOSIS: Depends on severity; most live into
adulthood
• PREVENTION: Folic Acid reduces incidence of neural
tube defects; (0.4mg/day) should be taken by all women of
childbearing age
PRENATAL DIAGNOSIS
•There are a variety of noninvasive and invasive techniques
available for prenatal diagnosis.
•Each of them can be applied only
during specific time periods
during the pregnancy for greatest
utility.
•The techniques employed for
prenatal diagnosis include:
–
–
–
–
–
–
–
Ultrasonography
Amniocentesis
Chorionic villus sampling
Fetal blood cells in maternal blood
Maternal serum alpha-fetoprotein
Maternal serum beta-HCG
Maternal serum estriol
PRENATAL DIAGNOSIS
PRENATAL DIAGNOSIS
•Ultrasonography
–This is a non-invasive
procedure that is harmless
to both the fetus and the
mother.
– The developing embryo
can first be visualized at
about 6 weeks gestation.
–Recognition of the major
internal organs and
extremities to determine if
any are abnormal can best
be accomplished between
16 to 20 weeks gestation.
PRENATAL DIAGNOSIS
• An ultrasound examination can
be quite useful to (Indication):
•
•
•
•
•
•
•
•
Multiple gestations are present
Determine the size and position of
the fetus,
Fetal age and growth
The size and position of the
placenta, and umbilical blood
flow.
The amount of amniotic fluid,
The appearance of fetal anatomy,
there are limitations to this
procedure.
Status of the uterine
environment, including the
amount of amniotic fluid
Presence or absence of
congenital anomalies: A good
example of this is Down syndrome
(trisomy 21), such as nuchal
thickening.
Ultrasonography
•Fetal age and growth are assessed by crown-rump length
during the 5th to 10th weeks of gestation. A
•fter that, a combination of measurements; including the
biparietal diameter (BPD) of the skull, femur length, and
abdominal circumference are used
•Congenital malformations that can be determined by ultrasound
include:
–The neural tube defects anencephaly and spina bifida;
–Abdominal wall defects, such as omphalocele and gastroschisis;
–Heart and facial defects, including cleft lip and palate.
Maternal Serum Screening
•A search for biochemical markers of fetal
status led to development of maternal
serum screening tests.
•One of the first of these tests assessed
serum a-fetoprotein (AFP) concentrations.
•AFP is produced normally by the fetal liver,
peaks at approximately 14 weeks, and
“leaks” into the maternal circulation via the
placenta. Thus, AFP concentrations
increase in maternal serum during the
second trimester and then begin a steady
decline after 30 weeks of gestation.
•In cases of neural tube defects and
several other abnormalities, including
omphalocele, gastroschisis, bladder
exstrophy, amniotic band syndrome,
sacrococcygeal teratoma, and intestinal
atresia, AFP levels increase in amniotic
fluid and maternal serum.
•In other instances, AFP concentrations
decrease, as, for example, in Down
syndrome, trisomy 18, sex chromosome
abnormalities, and triploidy.
•These conditions are also associated with
lower serum concentrations of human
chorionic gonadotropin (hCG) and
unconjugated estriol.
PRENATAL DIAGNOSIS
•Amniocentesis
•This is an invasive procedure in which a
needle is passed through the mother's lower
abdomen into the amniotic cavity inside the
uterus.
•Enough amniotic fluid is present for this to be
accomplished starting about 14 weeks
gestation.
•For prenatal diagnosis, most amniocenteses
are performed between 14 and 20 weeks
gestation.
•Within the amniotic fluid are fetal cells
(mostly derived from fetal skin) which can be
grown in culture for chromosome analysis,
biochemical analysis, and molecular biologic
analysis.
•In the third trimester of pregnancy, the
amniotic fluid can be analyzed for
determination of fetal lung maturity. This is
important when the fetus is below 35 to 36
weeks gestation, because the lungs may not
be mature enough to sustain life.
Amniocentesis
•During amniocentesis, a needle is inserted
transabdominally into the amniotic cavity
and approximately 20 to 30 mL of fluid is
withdrawn.
•Because of the amount of fluid required, the
procedure is not usually performed before
14 weeks gestation, when sufficient
quantities are available without endangering
the fetus.
•The risk of fetal loss as a result of the
procedure is 1%, but it is less in centers
skilled in the technique.
•The fluid itself is analyzed for biochemical
factors, such as AFP and
acetylcholinesterase.
•In addition, fetal cells, sloughed into the
amniotic fluid, can be recovered and used
for metaphase karyotyping and other
genetic analyses.
•With special stains (Giemsa) and highresolution techniques, chromosome banding
patterns can be determined.
•Furthermore, now that the human genome
has been sequenced, more sophisticated
molecular analyses using polymerase
chain reaction (PCR) and genotyping
assays will increase the level of detection
for genetic abnormalities.
Chorionic Villus Sampling
•Chorionic villus sampling (CVS)
involves inserting a needle
transabdominally or transvaginally into
the placental mass and aspirating
approximately 5 to 30 mg of villus
tissue.
•The risk of fetal loss from CVS is
approximately twofold greater than with
amniocentesis, and there have been
indications that the procedure carries
an increased risk for limb reduction
defects.
•Generally, these prenatal diagnostic
tests are not used on a routine basis
•Indications for using the tests include
the following:
–(1) advanced maternal age (35 years
and older);
–(2) previous family history of a genetic
problem, such as the parents having had
a child with Down syndrome or a neural
tube defect;
–(3) the presence of maternal disease,
such as diabetes;
–(4) an abnormal ultrasound or serum
PRENATAL DIAGNOSIS
•Chorionic Villus Sampling (CVS)
•In this procedure, a catheter is passed
via the vagina through the cervix and
into the uterus to the developing
placenta under ultrasound guidance.
•The introduction of the catheter allows
sampling of cells from the placental
chorionic villi.
•The most common test employed on
cells obtained by CVS is chromosome
analysis to determine the karyotype of
the fetus.
•The cells can also be grown in culture
for biochemical or molecular biologic
analysis.
•CVS can be safely performed between
9.5 and 12.5 weeks gestation.
PRENATAL DIAGNOSIS
•Maternal blood sampling for fetal
blood cells
•This is a new technique that makes use
of the phenomenon of fetal blood cells
gaining access to maternal circulation
through the placental villi.
•The fetal cells can be sorted out and
analyzed by a variety of techniques to
look for particular DNA sequences, but
without the risks that these latter two
invasive procedures inherently have.
PRENATAL DIAGNOSIS
•Triple" or "Quadruple"
screen
•Combining the maternal
serum assays may aid in
increasing the sensitivity and
specificity of detection for
fetal abnormalities.
•The classic test is the “triple
screen” for
•alpha-fetoprotein (MSAFP),
•beta-HCG,
•estriol (uE3).
•The "quadruple screen" adds
inhibin-A.