2.1 To determine the roles of Se and selenoproteins in ER stress
advertisement

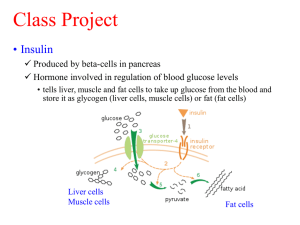
Selenoproteins and Selenium-dependent Redox Signaling Alter Diabetes Risk Dmitri Fomenko Department of Biochemistry and Redox Biology Center University of Nebraska-Lincoln Selenium is essential for human health Selenium deficiency may cause • Increased risk of cancer • Type 2 diabetes • Increased risk of AIDS in HIV-infected individuals • Male reproduction problems • Increased risk of viral infection • Keshan, Kashin-Beck diseases Selenium overnutrition may cause type 2 diabetes Selenium – link to cancer and diabetes Se has been shown to be effective in reducing the incidence of cancer in animal models and human clinical trials. Of the more than 100 reported studies that have examined the chemopreventive potential of Se, approximately two-thirds demonstrated a protective effect, and the remaining studies reported no effect. A interesting and unexpected side effect of Se supplementation was demonstrated for Nutritional Prevention of Cancer (NPC) and SELECT trials. NPC showed about a 50% increase in incidence of type 2 diabetes in the Sesupplemented group, which was associated with the high initial Se levels in plasma. The SELECT trial demonstrated high incidence of type 2 diabetes in men taking Se supplements. This observation was used as one of the reasons for trial termination. Taken together, these observations suggest that Se supplementation of people with high initial plasma Se may significantly increase the risk of diabetes development. A subsequent study showed that both, high and low Se supplementation is associated with hyperinsulinemia and decreased insulin sensitivity. The molecular mechanisms for the effect of dietary Se on the development of type 2 diabetes are not understood. • Se is present in proteins in the form of 21st amino acid – selenocysteine. More than 90% of the Se pool in mammals is exist in selenocysteine form • Selenocysteine is encoded by UGA codon, however, UGA is also a stop signal • mRNA structure, selenocysteine insertion sequence (SECIS) element, is essential for insertion of selenocysteine Selenocysteine insertion in eukaryotes Sec EFsec SBP2 AUG UGA UAA/UAG Selenocysteine versus Cysteine Selenocysteine Cysteine UGA codon pKa = 5.2 UGU, UGC-codons pKa = 8.3 Se- S S- S S S Sec serves redox function! Selenoproteomes Eukaryotes: 0-57 (Aureococcus) Bacteria: 0-57 (worm symbiont) Archaea: 0-9 (M. maripaludis) Viruses: 0-1 (fowlpox virus) Model organisms Mouse, rat Zebrafish Drosophila C. elegans S. cerevisiae, Arabidopsis Selenoproteins 24 37 3 1 0 Human Selenoproteome – 25 selenoproteins Selenoprotein GPX1 GPX2 GPX3 GPX4 GPX6 SelP DI1 DI2 DI3 SelW SelV SelT SelH SelM Sep15 TR1 TR2 TR3 SelS SelK SelO SelI SelN SelR SPS2 Sec-motif UxxT UxxT UxxT UxxT UxxT UxxC SxxU SxxU SxxU CxxU CxxU CxxU CxxU CxxU CxU U U U U U U U U UxxS UxC Protein secondary structure Selenoprotein expression and selenium supplementation 75Se metabolic labeling in HEK293 cells Stress response selenoproteins Gpx1, SelR, SelT, SelW kDa Housekeeping selenoproteins TR1, TR2, TR3, GPX4, TR1 Stress response selenoprotein expression is regulated by dietary selenium GPX1 GPX4 Sep15 SelW Novoselov et al, ARS, 2009 Available information regarding selenoproteins and diabetes 1. Glutathione peroxidase 1 (GPx1) Transgenic mice overexpressing Sec containing GPx1 develop obesity and insulin resistance phenotype. GPx1 knockout show low insulin level and high insulin sensitivity, however, positive effect of knockout was diminished by antioxidant supplementation. Gpx1 is a major hydroperoxides scavenger in mammalian cells. This selenoprotein account more than 90% of GPx activity in the cell and strongly regulated with Se. Thus, high Se is associated with high GPx1 activity, disrupted H2O2 signaling, and redox stress. 2. Selenoprotein S (SelS) SelS association with type 2 diabetes in an animal model was demonstrated. SelS is down-regulated in liver, adipose tissue, and skeletal muscle in the fed state of diabetic animal models in comparison with healthy animals. SelS was found to be insulin regulated and its expression was increased after insulin stimulation of human adipocytes. The goal of this project is to investigate the roles of Se and selenoproteins regulation of glucose homeostasis Considering Se function in the context of thiol oxidoreductases, we suggests that redox processes play a critical role in glucose homeostasis. We suggest that insulin signaling pathways are crosslinked with hydrogen peroxide-mediated signaling and selenoproteins, known as a subgroup of thiol oxidoreductases and contribute to the insulin signaling pathway by controlling the hydrogen peroxide concentration and redox homeostasis in the cell. Proposed project will be the first attempt to explain regulation of glucose homeostasis by Se, and the first attempt to explain mechanisms of the redox control of insulin signaling by selenoproteins. CENTRAL HYPOTESIS High dietary Se Low dietary Se Low glutathione peroxidases activity Oxidative stress, high H2O2 High glutathione peroxidases activity Redox stress, disrupted H2O2 mediated signaling PTPs, unknown mechanism unknown mechanism Hyperinsulinemia and insulin resistance – type 2 diabetes Overloaded b-cells and ER stress Unfolded protein response (Sep15, SelM, SelS, SelK) Cells adapt - homeostasis Type 1 diabetes Specific Aim 1: Clarify the role of Se and selenoproteins in type 2 diabetes development. This aim will be accomplished through completion of two sub-aims: 1.1 To investigate the role of Se and selenoproteins in glucose homeostasis. We will utilize several knockout and transgenic models to demonstrate contribution of different selenoproteins in type 2 diabetes development. 1.2 To determine mechanistic insides of Se and selenoproteins in insulin signaling. The roles of Se containing thiol peroxidases in insulin signaling will be addressed. 1.1 To investigate the role of Se and selenoproteins in glucose homeostasis. Experimental Design. We will employ transgenic/knockout mouse models, in which expression of either stress-related selenoproteins or the entire selenoprotein class is affected in the skeletal muscle of animals receiving a normal supply of dietary Se. In addition, we will estimates glucose homeostasis in GPx1 KO mouse. Type of animals Genotype Conditional knockout (skeletal muscle myocites) ΔSec tRNA Transgenic IA Knockout ΔGPx1 6 Description No Sec tRNA expression in skeletal muscle myocites , and consequently no selenoprotein expression in this cell type, selenoprotein expression in other cell types is not affected Overexpression of Sec tRNA transgene, low levels of stress-related selenoproteins (e.g., GPx1); expression of essential selenoproteins is not affected No GPx1 expression, increased insulin sensitivity, low level of insulin and resistance to high fat diet Skeletal muscle conditional Sec tRNA KO model will be generated using floxed Sec tRNA transgenic mouse. Groups of seven male animals of this model with control will be subjected to six different diets (Harland TekLad) containing 0 parts per million Se (Se-deficient), 0.1ppm Se and 0.4ppm Se, and a similar custom made diet based on a high fat diet TD.06414. The same groups of animals will be subjected to the same diets with additional supplementation with 20mM NAC in water. Glucose homeostasis parameters. After one month of dietary supplementation we will start weekly measurement of fasting or fed state plasma glucose, Se, and insulin levels. Glucose tolerance tests will be performed in 12 hour fasted animals by injection of glucose followed by plasma glucose measurements every 60 minutes. The Se level in the plasma will be estimated using the ICPMS. Insulin sensitivity tests will be performed on 12 hour fasted animals by intraperitoneal injection of insulin. Plasma glucose will be measured every 60 minutes. Selenoprotein expression will be estimated by Western Blot and 75Se metabolic labeling. All animals will be euthanized 18 weeks after beginning the experiment and tissues will be collected for redox parameters measurement. Similar experiment will be performed with I6A and GPx1 KO animal model. All collected samples will be analyzed for redox stress parameters. Expected Outcomes of Specific Aim 1.1. We expect to observe type 2 diabetes-like phenotype for both the Sec tRNA KO and transgenic models at all Se concentrations. We expect this phenotype will be more pronounced for a high fat diet. NAC supplementation will partially compensate Se deficiency in KO models according to our central hypothesis. Control groups will show type 2 diabetes-like phenotype for both a low and high Se diet. This experiment will help us determine the contribution of housekeeping and stress response selenoproteins in glucose homeostasis. The experiment on the GPx1 knockout model will allow us to identify additional selenoproteins that are involved in diabetic phenotype development. 1.2 To Determine mechanistic insides of Se and selenoproteins in insulin signaling. We demonstrated the roles of thiol peroxidases in H2O2 mediated signaling using yeast system. Thiol peroxidases oxidize regulatory and signaling proteins, resulting in transcriptional response and signaling programs (Fomenko et al, PNAS, 2011). Thioredoxin reductases Peroxiredoxins Thioredoxins Kinases NADPH Signaling Transcription factors H2O2 Other targets Glutathione reductase Glutaredoxins Glutathione peroxidases Glutathione Protein tyrosine phosphatases, including PTP1, could be reversibly inactivated by H2O2 oxidation. PTP1 is responsible for control of phosphorylation status of the insulin receptor. Insulin stimulation of mammalian cells is associated with Nox4-mediated H2O2 production, which causes reversible oxidization and inhibition of PTP1B activity and promotes insulin signaling. Recombinant PTP1 oxidation was demonstrated at 75mM of H2O2 and higher; however, mammalian cells induce apoptosis at such concentrations. Considering our observation for yeast cells, we hypothesize that PTP1 oxidation is mediated by thiol peroxidases – GPxs and/or PRxs and may be regulated by Se. We suggest that mammalian GPxs, other than GPx1, and PRxs mediate PTP1 oxidation in insulin signaling pathways. Experiment Design. 1. The HEK293 cell line is established model system and will be used for insulin signaling studies. We will use Dharmacon siGENOME SMARTpool siRNAs to knockdown GPxs and PRxs. The cells will be transfected with siRNAs and treated with 10mM H2O2 after two days. Phosphorylation status of insulin receptor b-subunit will be estimated by Western Blot with phospho-specific antibodies. 2. In addition, we will estimate efficiency of oxidation of recombinant PTP1 protein with H2O2 and oxidized recombinant GPx3 from yeast. 3. Phosphorylation status of insulin receptor b subunit will be estimated for miocytes of animal models from Sub-aim 1.1 Expected Outcomes of Specific Aim 1.2 We will determine the contribution of Sec containing GPxs in insulin receptor regulation through reversible oxidation of protein tyrosine phosphatases. We expect that thiol peroxidases, other than GPx1, are involved in the transferring of oxidative equivalent from H2O2 to PTP1. This process may be regulated by Se supplementation. Specific Aim 2: Characterize the role of Se and selenoproteins in the protection of pancreatic b-cells from ER stress and oxidative stress. This aim will be accomplished through completion of two sub-aims: 2.1 Determine the role of Se and selenoproteins in ER stress defense. We expect to determine how Se supplementation influences ER stress response and protects pancreatic b-cells from ER stress-mediated apoptosis. 2.2 Investigate the protective role Se and selenoproteins play in oxidative protein folding induced oxidative stress. The contribution of different selenoproteins in protection from protein folding associated oxidative stress in pancreatic b-cells will be addressed. 2.1 To determine the roles of Se and selenoproteins in ER stress defense. ER is characterized by very intense and diverged redox processes that are catalyzed by numerous thiol oxidoreductases and selenoproteins. Seven of the 25 identified human selenoproteins are localized in ER. Hyperinsulinemia is associated with increased insulin synthesis and secretion. Pancreatic bcells overload, resulting in ER stress and activation of the ER stress-mediated apoptosis pathway. b-cells death is one of the central features in conversion of type 2 diabetes to type 1. ER selenoproteins are regulated by Se supplementation and serve in protein folding and ER stress related pathways. Thus, Se may contribute in pancreatic b-cells protection from ER stress and delay or prevent apoptosis. Translocon Export Secretory pathway Protein glycosylation Oxidative folding OST SelT, SelN, DI2 CYTOSOL Glycoprotein folding quality control Chaperones ER UGT, Sep15 SelM Retrotranslocation SelS, SelK Cytosolic degradation Experiment Design. b-cells conditional Sec tRNA KO model will be generated using floxed Sec tRNA transgenic mouse. Groups of seven male animals of this model and seven male animals of control groups will be subjected to the high fat diet TD.06414 (Harland TekLad) to initiate type 2 diabetes. After five month of dietary supplementation we will begin monthly measurement of glucose homeostasis parameters as described in Specific Aim 1.1 for control of diabetes progression. All animals will be euthanized after one year, pancreatic tissue will be collected, and expression of each ER selenoprotein will be analyzed with Western Blot using specific antibodies available in project leader’s laboratory. ER stress will be verified by the expression level of the BiP chaperone in all collected tissues using Western blot. The UPR will be analyzed by monitoring the phosphorylation status of eukaryotic initiation factor eIF2α. The EOR will be examined by monitoring the phosphorylation status of NF-κB. Similar analysis of control groups from Specific Aim 1.1. Expected Outcomes of Specific Aim 2.1 This experiment will help to determine contribution of Se and ER selenoproteins in ER stress protection. 2.2 Investigate the protective role Se and selenoproteins play in oxidative protein folding induced oxidative stress. Oxidative folding in the ER is associated with formation of H2O2 molecules for each formed disulfide bond. The insulin molecule contains three disulfides and its increased synthesis may be linked with strong oxidative stress with subsequent b-cells death. At the same time, b-cells have only 1% of catalase, 2% of GPx, and 29% of SOD activity compared to liver cells. In this experiment, we will estimate the roles of different selenoproteins in b-cells protection from oxidative stress associated death. Experiment Design. I6A transgenic model and corresponding control samples of b-cells from Specific Aim 1.1, and b-cells conditional Sec tRNA KO model samples will be analyzed for oxidative damage. Selenoprotein expression will be estimated by Western Blot and 75Se metabolic labeling. This experiment will allow us to address the contribution of housekeeping and stress related selenoproteins in b-cells protection from oxidative stress. Expected Outcomes of Specific Aim 2.2. We expect to observe a protective role of Se and ER selenoproteins in ER stress conditions. In addition, we expect that Se and selenoproteins are involved in b-cells protection from protein folding-mediated oxidative stress. Summary of expected outcome: 1. We will determine roles of Se and particular selenoproteins in glucose homeostasis control. 2. We will show the roles of Sec-containing glutathione peroxidases in insulin signaling pathways. 3. We will determine the roles of Se and selenoproteins in protection of pancreatic b-cells from ER stress and oxidative stress. Proposed project and NPOD mission Selenium is essential micronutrient in human diet. Our study will help to explore mechanisms of Se associated diabetes development and will help to redefine an optimal level of dietary Se, as well as the upper subtoxic limit, to provide health benefits in cancer prevention without risk of diabetes development. Current research projects and link to nutrigenomics: This project is logically based on my recent work. I have strong experience in selenium biology and more than 20 of my recent publications are related to identification and functional characterization of selenoproteins. I developed and characterized several selenoprotein knockout mouse models. One of my side-project is related to the boron metabolism and identification of the mechanisms of boron toxicity.