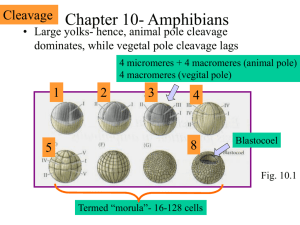
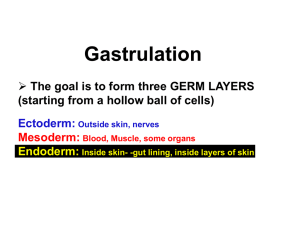
AxisForm.Gastrulation.2.11
advertisement

Axis Formation and Gastrulation II The Xenopus Oocyte is Asymmetric “Animal” VegT Vg1 Maternal Determinants “Vegetal” VegT: Transcription factor -Promotes “vegetal” identity (endoderm) -Activates mesoderm inducers (nodals/TGF-ß’s) Vg1: Another nodal/ TGF-ß’s Sperm Entry Point Determines D/V Axis in Xenopus “Animal” An Cortical Rotation D Wnt11 VegT Wnt11 “Vegetal” Maternal Determinants VegT: Transcription Factor Promotes “vegetal” identity (endoderm) Wnt11: Signaling Ligand Promotes Dorsal identity V VegT Veg Specifying the Germ Layers Mesoderm Inducers are TGF-ß Family Members (Vg1, Nodals (Xnr’s), Activin) -higher in dorsal mesoderm Wnt11 Specifying The Spemann-Mangold Organizer V Wnt11 Nodals (TGF-ß’s) D V V D D Transplant Dorsal Blastopore Lip Donor: Pigmented (newt) Host: Unpigmented (newt) Spemann and Mangold, 1924 V V D D Form Second Body Axis Spemann and Mangold, 1924 Normal Body Axis V V D D Second Body Axis Second Body Axis: Dorsal Mesoderm (Notocord)--Donor Cells Neural Plate--Host Cells Other mesoderm--mix of Donor and Host Donor tissue “organized” host tissue to take on new cell identities Blastopore lip = an Organizer Spemann and Mangold, 1924 Also two sites of gastrulation Specifying The Speman-Mangold Organizer V D V D wnt11 nodals The Organizer: -Patterns the mesoderm along the D/V axis -Determines the site of gastrulation -Allows for induction of the nervous system (neuroectoderm) Fate Map of the Xenopus Blastula Xenopus An V D Veg Hypothesis: Neural Induction The dorsal lip secretes a signaling molecule that patterns the mesoderm and induces neural plate specification Search for molecules that can mimic organizer activity Find secreted INHIBITORS of TGF-ß ligands (Chordin, Noggin, Follistatin) Neural is DEFAULT state and TGF-ß signaling is required to INDUCE ectoderm TGF-ß (BMP, Dpp) Frog Xolloid/Tolloid Fly D Chordin, Sog xolloid sog Note: BMP4 is different type of TGF-ß than nodals -nodals form early gradient that is high in dorsal regions -BMPs form later gradient that is high in ventral regions V Fish and Frog Embryos Appear Very Different Yolk Cell Fish and Frogs Do Things Similarly -The oocyte has an Animal-Vegetal Axis -The Wnt pathway initiates the D/V Axis (unclear how--doesn’t seem to be sperm) -BMPs and Wnts pattern the mesoderm V V D D BMPs V V nodals Organizer D(Shield) -Nodal (and FGF) signaling specifies the mesoderm (and endoderm) with a dorsal bias -Dorsal mesoderm makes the organizer (shield) D V Wnts D Fate Mapping (Lineage Tracing) to Investigate Cell Identity and Developmental Potential Xenopus An V D Veg Zebrafish It is not birth, marriage or death, but gastrulation, which is truly the most important time in your life. - Lewis Wolpert (1986) Gastrulation Cell Movements Relevant for Gastrulation Getting cells inside Spreading tissues out Moving cells around Making tissues longer Convergence/extension Xenopus Gastrulation Initiates at the Organizer (aka Dorsal Blastopore Lip) Xenopus Gastrulation Zebrafish Fate Map Schier and Talbot, 2005 Zebrafish Gastrulation Early Zebrafish Cleavages Zebrafish Epiboly Transforms the Embryo Into a Hemisphere Solnica-Krezel, 2006 Cells Involute and Ingress to Form the Dosal Shield Cells Migrate Anteriorly After Involution Solnica-Krezel, 2002 Convergence/Extension Also Contributes to A/P Axis Formation Extension of cells along the A/P axis Convergence of lateral cells toward the midline of the embryo Convergence/Extension Requires the PCP Pathway Solnica-Krezel, 2006 The Anterior-Posterior Axis is Also Coupled to Gastrulation The developmental potential and inducing properties of cells in the dorsal blastopore lip change with time: -Cells in the lip early become anterior mesoderm and induce anterior neural tissue -Latter cells become posterior and induce more posterior neural structures -A gradient of Wnt activity is high in posterior and low in anterior Chick Embryos Look Different but Act Similarly The Chick Embryo Forms as a Flattened Disc of Cells On the Yolk The primitive streak/node is the organizer and expresses goosecoid and chordin The posterior marginal zone initiates primitive streak/organizer formation and expresses Vg1 and Wnt8 The primitive streak elongates with the node/organizer at the leading edge Hensen’s Node is the Avian Organizer Similar to Xenopus blastopore and Fish dorsal shield in terms of both patterning and gastrulation movements The node can induce nervous system development and a secondary axis when transplanted The node expresses BMP antagonists, like the Xenopus and Zebrafish organizers All Vertebrate Embryos Have a Spemann-Mangold Organizer Organizer Bird Fish Dorsal Shield Frog Blastopore Lip Bird Henson’s Node Mouse Node Chick Gastrulation Movements -In the Node and Primitive Streak, cells delaminate from the epiblast and ingress to form the endoderm and mesoderm -Epiblast cells continue to enter the streak from lateral regions -Once they have ingressed in the streak, newly formed endoderm and mesoderm move laterally again Gastrulation and Patterning Follow the Same Logic as Fish and Frogs As with fish and frogs, the first cells gastrulating through the chick organizer (node) become anterior endoderm and prechordal plate mesoderm The next cells through will form notochord These first cells also induce the nervous system from the overlying ectoderm Cells gastrulating through more posterior primitive streak become other mesoderm and endoderm derivatives BUT, note that the organizer MOVES as the primitive streak first advances and then regresses More posterior regions of notocord are formed from cells migrating through node as it regresses The A/P Axis is “laid down” During Primitive Steak Regression This is in contrast to the active extension of the A/P axis seen in frog and fish embryos (although some active mechanisms do further elongate the chick A/P axis) Consequently, posterior development is delayed relative to anterior development “Laying down” the notocord during regression of Henson’s node Human Embryos Gastrulate Like Chick Embryos Different Embryos, Common Themes Activin (TGF-ß): A morphogen for the mesoderm Animal cap assay (After Green et al., 1992) (After Asashima, 1994) Lineage Analysis and Fate Mapping Goal: Identify which cells in the early embryo give rise to particular cells in the later embryo or adult Lineage Analysis and Fate Mapping Approaches: 1) Just watch closely Lineage Analysis and Fate Mapping Approaches: 1) Just watch closely 2) Cell transplantation 3) Use a Lineage Tracer -Inject into single cells or few cells -Activate in single cells or few cells Fate Mapping by Single Cell Injection of Lineage Tracer e.g. Woo and Fraser, 1995 Laser Activation of Lineage Tracer “Caged” fluorescein: e.g. Jopling and den Hertog, 2005 Photo-activatable GFP Absorbance spectrum before PA Absorbance spectrum after PA Patterson and Lippincott-Schwartz, 2002 Lineage Analysis and Fate Mapping Approaches: 1) Just watch closely 2) Cell transplantation 3) Use a Lineage Tracer -Inject into single cells or few cells -Activate in single cells or few cells 4) Genetic labeling -Random labeling -Labeling a specific lineage Genetic Mosaics Genetic mosaics can be created by mitotic recombination induced by X-rays or a site-specific recombinase Genetic Tracking of Specific Lineages Question: What do cells that express YFG at time X develop into? Actin Promoter YFG STOP GFP loxP CRE-ER Add tamoxofen at time X Cells expressing YFG at that time permanently labeled with GFP Some things to worry about: -Can you identify and label your cells of interest? -Does your labeling technique interfere with normal development? -Is your lineage tracer stable over time? -Is your lineage tracer diluted by cell division?