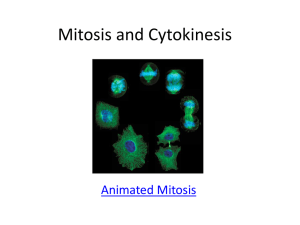
Cell Division and Mitosis
advertisement

Cell Division and Mitosis Quote “Although memorizing of the phases of mitosis has tortured students for decades, understanding how these events actually occur continue to occupy cell biologists, as a complete molecular model has yet to be obtained” S. Gadde and R. Heald Current Biology 14:R797-R805 (2004) The Cycle of Cell Growth and Division: An Overview The products of mitosis are genetic duplicates of the dividing cell Chromosomes are the genetic units divided by mitosis Mitotic Cell Division DNA replication Equal separation (segregation) of replicated DNA molecules Delivery to daughter cells Two new cells, same information as parent cell Mitosis Mitosis is the basis for Growth and maintenance of body mass in multicelled eukaryotes Reproduction of many single-celled eukaryotes Chromosomes DNA of eukaryotic cells is divided among individual, linear chromosomes Located in cell nucleus Ploidy of a cell or species Diploid (2n) Haploid (n) Eukaryotic Chromosomes Eukaryotic cells contain 2 sets of chromosomes = homologous pairs Diploid – 2 sets of chromosomes Autosomal cells Haploid – 1 set of chromosomes Reproductive cells (egg, sperm) Eukaryotic Chromosomes Fig. 10-2, p. 203 Sister Chromatids DNA replication and duplication of chromosomal proteins produces two exact copies (sister chromatids) Chromosome cell division segregation occurs during The Mitotic Cell Cycle Interphase extends from the end of one mitosis to the beginning of the next mitosis After interphase, mitosis proceeds in five stages Cytokinesis completes cell division by dividing the cytoplasm between daughter cells The mitotic cell cycle is significant for both development and reproduction Mitosis varies in detail, but always produces duplicate nuclei Mitotic Cell Cycle Includes Mitosis mitosis and interphase occurs in five stages Prophase Prometaphase Metaphase Anaphase Telophase The Cell Cycle Fig. 10-3, p. 203 Checkpoints ** Interphase Fig. 10-4a (1), p. 204 Fig. 10-4b, p. 205 Stage 1: Prophase Chromosomes Spindle condense into short rods forms in the cytoplasm Prophase Fig. 10-4a (2), p. 204 Stage 2: Prometaphase Nuclear envelope breaks down Spindle enters former nuclear area Sister chromatids of each chromosome connect to opposite spindle poles Kinetochore of each chromatid attaches to the spindle microtubules Prometaphase Fig. 10-4a, p. 204 Spindle Connections at Prometaphase Fig. 10-6, p. 206 Prometaphase chromosome Sister chromatid I Sister chromatid II Kinetochore I Spindle pole Kinetochore II Spindle microtubules Spindle pole Fig. 10-6, p. 206 Stage 3: Metaphase Spindle is fully formed Chromosomes align at metaphase plate Moved by spindle microtubules Metaphase Fig. 10-4b, p. 204 Stage 4: Anaphase Spindle separates sister chromatids and moves them to opposite spindle poles Chromosome segregation is complete Anaphase Fig. 10-4b, p. 204 Stage 5: Telophase Chromosomes decondense Return to extended state typical of interphase New nuclear envelope forms around chromosomes Telophase Fig. 10-4b, p. 204 Mitosis Fig. 10-5, p. 206 Cytokinesis Division of cytoplasm completes cell division Produces two daughter cells Each daughter nucleus produced by mitosis Cytokinesis in Animal Cells Proceeds by furrowing Band of microfilaments just under the plasma membrane contracts Gradually separates cytoplasm into two parts Cytokinesis by Furrowing Fig. 10-8, p. 208 Plant Cytokinesis Cell wall material is deposited along the plane of the former spindle midpoint Deposition continues until a continuous new wall (cell plate) separates daughter cells Cytokinesis by Cell Plate Formation Fig. 10-9, p. 208 Chromosomes in the Cell Cycle At what part of the cell cycle would you see a chromosome that looks like this? Give your reasons. Rate of Mitosis If you were to examine a sample of connective tissue cells from a small but adult mammal and a second sample from a fetus of the same species, in which would you expect to find more cells undergoing mitosis? Why? Formation and Action of the Mitotic Spindle Animals and plants form spindles in different ways Mitotic spindles move chromosomes by a combination of two mechanisms Spindle Formation In In animal cells Centrosome divides, the two parts move apart Microtubules of the spindle form between them plant cells with no centrosome Spindle microtubules assemble around the nucleus Centrosome and Spindle Formation Fig. 10-10, p. 210 In the Spindle Kinetochore microtubules Run from poles to kinetochores of chromosomes Nonkinetochore microtubules Run from poles to a zone of overlap at the spindle midpoint without connecting to chromosomes A Fully Developed Spindle Fig. 10-11, p. 210 During Anaphase Kinetochores move along kinetochore microtubules Pulling chromosomes to the poles Nonkinetochore microtubules slide over each other Pushing the poles farther apart Anaphase Spindle Movements Fig. 10-12, p. 211 Kinetochore Movement Fig. 10-13, p. 211 Current Biology 14:R797-R805 (2004) Latest research Science 311:388-391 (2006) Kapoor Lab Cell Cycle Regulation Cyclins Internal controls that directly regulate cell division Internal and cyclin-dependent kinases checkpoints Stop cell cycle if stages are incomplete External controls Coordinate mitotic cell cycle of individual cells within overall activities of the organism Control of the cell cycle is based on cyclically activated protein kinases Phosphorylation of key proteins that regulate DNA replication (S phase), Mitosis (M phase), and Cytokinesis CDK Cyclin/CDK Phosphorylation Cell Cycle Control Complexes of cyclin and a cyclindependent protein kinase (CDK) Directly control cell cycle CDK Is activated when combined with a cyclin Adds phosphate groups to target proteins, activating them Cell Cycle Control Activated proteins trigger the cell to progress to the next cell cycle stage Each major stage of the cell cycle Begins with activation of one or more cyclin/CDK complexes Ends with deactivation of complexes by breakdown of cyclins Cyclin/CDK Control Fig. 10-15, p. 214 Cyclin B Cyclin B CDK1 G2-to-M checkpoint CDK1 Cyclin E G2-to-S checkpoint Cyclin E CDK2 CDK2 Stepped Art Fig. 10-15, p. 214 Human cells CDK and cyclin Internal Controls Important internal controls create checkpoints Ensure that the reactions of one stage are complete before cycle proceeds to next stage External Controls Based on surface receptors that recognize and bind signals Peptide hormones and growth factors Surface groups on other cells Molecules of the extracellular matrix Binding triggers internal reactions that speed, slow, or stop cell division Cancer is a genetic disease Accumulation of mutations in key regulatory genes resulting in abnormal growth control Metastatic lung tumors in a human liver Genomic Instability Loss of growth control results in genomic instability Genomic Instability Science 297:544 (2002) Cancer Control of cell division is lost Cells divide continuously and uncontrollably Form rapidly growing mass of cells that interferes with body functions Cancer cells break loose from their original tumor (metastasize) Form additional tumors in other parts of the body Tumor Cells Fig. 10-16, p. 215 Human cells CDK and cyclin Targeted drugs: Tyrosine kinase inhibitors Herceptin for the treatment of metastatic breast cancer Gleevec (imatinib mesylate) for the treatment of chronic myelogenous leukemia (CML) Avastin (trastuzumab) (bevacizumab) for the treatment of metastatic colorectal cancer Targeted Therapies Her-2/neu as breast cancer target HER2 (human EGFR-related gene) Amplified up to 100X in tumor cells of about 30% of patients with invasive breast cancer Significant clinical correlation between gene amplification and reduced survival Herceptin Therapeutic monoclonal antibody (mAb) Targeted drugs: Tyrosine kinase inhibitors Herceptin for the treatment of metastatic breast cancer Gleevec (imatinib mesylate) Novartis for the treatment of chronic myelogenous leukemia (CML) Avastin (trastuzumab) Genentech (bevacizumab) Genentech for the treatment of metastatic colorectal cancer Targeted Therapies Gleevec Bcr-abl kinase Constitutively active cytoplasmic tyrosine kinase Present in virtually all patients with CML activates signal transduction pathways leading to uncontrolled cell growth Gleevec blocks ATP binding to the kinase Gleevec resistance – 20% of patients who take Gleevec become resistant to it within 3 years Bcr-abl mutations in the ATP binding pocket of the kinase domain 15 Targeted drugs: Tyrosine kinase inhibitors Herceptin for the treatment of metastatic breast cancer Gleevec (imatinib mesylate) Novartis for the treatment of chronic myelogenous leukemia (CML) Avastin (trastuzumab) Genentech (bevacizumab) Genentech for the treatment of metastatic colorectal cancer Targeted Therapies Angiogenesis New blood vessel formation Dr. Judah Folkman Avastin Binds to VEGF (vascular endothelial growth factor) Future New diagnostic tools (cellular/gene based testing) -predictors of which treatment works best on which person Nature 415:532 (2002) Future Combinatorial therapies Combine an anti-EGFR mAb and tyrosine kinase inhibitor within the cell Erbitux (ImClone) with Tarceva (Genentech) Future “Promiscuous” drugs SU11248 (Pfizer) Interferes with several signaling pathways Anti-tumor and antiangiogenic properties Inhibits a variety of kinase receptors Cell Division in Prokaryotes Replication occupies most of the cell cycle in rapidly dividing prokaryotic cells Replicated chromosomes are distributed actively to the halves of the prokaryotic cell Mitosis has evolved from binary fission Replication in Prokaryotes Begins at origin of replication of the bacterial chromosome Reactions catalyzed by enzymes in middle of cell Once the origin of replication is duplicated Two origins migrate to two ends of the cells Cytoplasmic Division A partition of cell wall material grows inward until the cell is separated into two parts Bacterial Cell Division Fig. 10-17, p. 216 Video: Mitosis overview