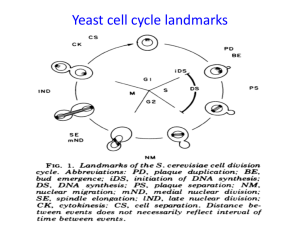
fission yeast
advertisement

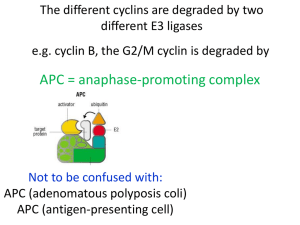
Lecture 8 Getting into and out of mitosis 2001 who won the Nobel prize?? Outline: Uncovering the cyclin/CDK paradigm G2/M Exiting M-phase Paper: Anaphase regulation in budding yeast M-phase is induced by a soluble factor 70’s cell fusion experiments Factor in mitotic cell induces premature mitosis in interphase cell M premature chromosome condensation is a disaster! S the cell cycle must be highly coordinated. What is M-phase inducer? 3 lines of experimental approach: yeast Xenopus eggs invertebrate eggs YEAST Advantages: rapidly growing, divides every 90 min powerful genetics cell cycle progress easy to follow Lee Hartwell S. cerevisiae Paul Nurse S. pombe Disadvantages: Different from higher eukaryotes nuclear envelope does not break down in M-phase spindle assembly occurs during DNA replication (budding yeast) little or no chromosome condensation in M-phase Note on yeast nomenclature S. cerevisiae (budding yeast) wild type or dominant mutations protein CDC28 cdc28-4ts, ∆cdc28 Cdc28 S. pombe (fission yeast) wild type or dominant mutations protein cdc2+ cdc2D cdc2ts cdc2Cdc2 S. pombe = fission yeast Fission yeast must reach a critical size to enter mitosis Approach Identify temperature sensitive mutants that block the cell cycle at a specific stage (size) asynchronous population most arrest at random cell cycle states cdc mutant “cell division cycle” Cdc2 is the master regulator of cell division WT always divide at the same size cdc2(ts) loss of function cdc2D gain of function “wee” essential, no other mutation can rescue loss of cdc2 Mitosis-inducing activity of Cdc2 is inhibited by Wee1 and stimulated by Cdc25 WT cdc2ts cdc25ts wee1ts cdc25ts, wee1ts cdc2D, wee1ts mitotic catastrophe Cdc2 - protein kinase Wee1 - protein kinase Cdc25 - protein phosphatase WT 5X cdc2+ 3X wee1+ 5X cdc25+ can confirm interactions by overexpression: Conclusions: Cdc2 is the master regulator of cell division in fission yeast Cdc25 is a positive regulator of Cdc2 Wee1 is a negative regulator of Cdc2 These genes are highly conserved in all eukaryotes S. cerevisiae cdc2 homologue = CDC28 human gene also complements ~ 65% identical Invertebrate and Xenopus eggs sea urchin, starfish, clam Advantages: stripped down cell cycle: M alternating S- and M-phases no G1 or G2 phases synchronous divisions easy to inject good for biochemistry S Eggs divide without growing somatic cells embryonic cells 12 embryonic cell cycles typical somatic cell cycle G1 0 3 S 6 9 12 G2 15 18 M 21 Hours Observation: protein synthesis is required for early embryonic divisions 24 Identification of “cyclins” sea urchin eggs fertilize eggs synchronous rounds of division incubate with 35S - methionine take sample every 10 min SDS-PAGE and autoradiography newly synthesized proteins get labeled Tim Hunt, Joan Ruderman mitosis interphase mitosis interphase cyclin A cyclin B other proteins cyclin B abundance I M I M cell cycle state Conclusion Cyclin synthesis and destruction correlates with progression through the cell cycle cyclins are potential regulators Xenopus laevis oocyte maturation diploid meiosis fertilization haploid 1 mm diameter! cleavage diploid Discovery of MPF = Maturation Promoting Factor Masui and Markert 1971 a more general role..... MPF = M-phase Promoting Factor also present in mitotic somatic cells KEY EXPERIMENT - Masui, Lohka and Maller biochemical purification of MPF 1 liter of frog eggs 1 mg using oocyte maturation as an assay? no. monitored nuclear envelope breakdown of pronuclei in egg extract MPF has two subunits: p34 p46 complex has kinase activity toward histone H1 VERY FAMOUS WESTERN BLOT: 1988 antibody to conserved region of cdc2: “PSTAIRE” p34 = cdc2 p46 = cyclin B M-phase promoting factor = MPF =cdk1/cyclinB protein kinase complex, 2 subunits: 1) cdk1 cyclin dependent kinase 1 = yeast cdc2 • Induces mitosis by phosphorylating specific downstream targets on serine and threonine 2) cyclin B • regulatory subunit that activates cdk1 • abundance oscillates during the cell cycle cell cycle paradigm: transitions are regulated by cdk/cyclin complexes In yeast: single cdk, many cyclins • Cyclin B protein is synthesized continuously • Threshold cyclin B level induces MPF kinase activity • Cyclin B disappears suddenly during anaphase Experiments to prove that cyclin synthesis drives the embryonic cell cycle electric shock simulates fertilization in vitro system Marc Kirschner Andrew Murray cell cycle reconstitution in vitro low MPF low histone H1 kinase activity high MPF high histone H1 kinase activity cyclin B synthesis can drive the cell cycle is cyclin B necessary to drive the cell cycle? specifically degrade cyclin B mRNA: cyclin B is both necessary and sufficient Is cyclin B destruction required to exit mitosis? add mRNAs to extract: YES Other post-translational steps control MPF activation Why the lag if enough cyclin B has been synthesized? Other evidence for post-translational regulation fission yeast - Cdc13 (cyclin B) overexpression does not affect cell cycle: Cdc25 and Wee1 regulate clams and starfish oocyte maturation preMPF MPF, no protein synthesis Drosophila embryo limiting protein is Cdc25, not cyclin B Phosphorylation on different cdk sites can either stimulate or inhibit MPF activity mutational analysis of cdc2 in yeast: Tyrosine 15 (Y-15) phosphorylation inhibits Cdc2 Threonine 161 (T-161) phosphorylation activates Cdc2 e.g. mutate tyrosine-15 to phenylalanine wee phenotype Wee1 Mik1 Y15 kinases Cdc25 Y15 ppase MPF activity controlled by: 1) cyclin B levels 2) phosphorylation of cdk1 Multiple levels of regulation provide input for “checkpoint control” Feedback mechanisms: When MPF level reaches threshold irreversible activation High MPF levels trigger cyclin B degradation irreversible inactivation Exit from mitosis Ubiquitin-mediated proteolysis Enzymes in ubiquitin pathway E1: activates ubiquitin only 1 gene in yeast E2: acquires ubiquitin through high energy thioester linkage = ubiquitin-carrier or conjugating enzyme 11 genes in yeast E3: catalyzes attachment of ubiquitin to protein substrate = ubiquitin ligase binds E2 and substrate, like a scaffold can be in large regulatory complex many versions, provides specificity rate-limiting step!! proteosome EM E3 ligase for cyclin B = Anaphase Promoting Complex (APC) identified by fractionating egg extracts, yeast genetics 8 subunits degrades other proteins besides cyclin B that are required for anaphase and mitotic exit What are the APC targets? mitotic cyclins Pds1 - chromosome cohesion regulator Xkid ?? temporally specific proteolysis: orderly and irreversible sequence of events What generates specificity of APC? Accessory factors: recognize destruction box Cdc20 - activates APC earlier targets Pds1, mitotic cyclins Cdh1 - activates APC later targets more cyclins, APC subunits ENTRY INTO INTERPHASE