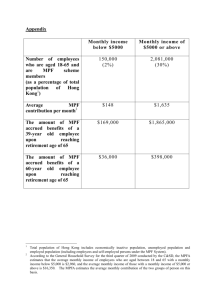
cdc2
advertisement

Lectures 21 and 22: The regulation and mechanics of cell division • Today - cell cycle (regulation of cell division) – Cell proliferation – The eukaryotic cell cycle – Measuring the cell cycle – Models of the cell cycle: from fungi to frogs – The cell cycle is regulated by cyclin-dependent kinases • Next time - mechanisms of cell division A cell cycle is one round of growth and division Cells only come from pre-existing cells cytokinesis mitosis Growth and division must be carefully regulated Unregulated cell growth = cancer The eukaryotic cell cycle is partitioned into four “phases” Division occurs in “M-phase:” “mitosis” and “cytokinesis” (<1 hr) 4C 4C 2C (DNA replicated, diploid chr #) Most cell growth occurs during “G1” (6-20+ hrs; duplicate organelles, double in size) DNA replication occurs during “S-phase” (4-10+ hrs)… “G2” prepares cells for division (1-6+ hrs)… 2C 4C ECB 18-2 2C (unreplicated DNA, diploid chr #) G1+S+G2=“Interphase” Division = “M-phase” A “typical” cell cycle for animal cells is 24-48 hrs long, but varies… Cell cycle times vary Can determine phase of cell cycle from DNA content Number of cells Cells in G1 Adapted from MBoC figures 17-5 and 17-6 Where are cells in G1, S, G2 and M on plot? Which phase has most cells in it? Lasts longest? Cells in G2/M Cells in S 1 2 DNA content (arbitrary units) ECB 18-2 Transition from one phase to another is triggered We will take a historical perspective to ‘triggers’ Regulating the eukaryotic cell cycle: studies in four model organisms • Marine invertebrates: – Surf clam (Spisula) See HWK 618-619 – Sea urchins and starfish • Frog eggs and embryos: – Rana pipiens (Northern leopard frog) – Xenopus laevis (African clawed frog) • Cultured cells – HeLa (Human cervical carcinoma) • Yeast cell division cycle (“cdc”) mutants: – Saccharomyces cerevisiae “budding” yeast – Schizosaccharamyces pombe “fission” yeast 1. Fission yeast “cell division cycle (cdc)” mutants define a master regulator (tigger) of the G2/M transition “Wild-type” fission yeast WT cdc2- (loss of function) “cdc” WEE2 = cdc2D (gain of function) Mutant “wee” Phenotype wee1- (loss of function) wee cdc13- (loss of function) cdc cdc25- (loss of function) cdc wee1 cdc25 G2 cdc2 cdc13 Genetic pathway M 2. Frogs: unfertilized eggs contain an M-phase Promoting Factor Transfer M-phase cytoplasm to interphase oocyte… ECB 18-9 Control expt; Transfer interphase cytoplasm to interphase cell - no effect Nucleus Egg in “M-phase” Oocyte in “interphase” Oocyte “matures” (enters M-phase)… Transfer of cytoplasm from egg to oocyte induces M-phase: “M-phase promoting factor (MPF)” Not restricted to egg cytoplasm - Any M-phase cytoplasm will induce M-phase ECB figure 18-5 MPF activity cycles during the cell division cycle M-phase Interphase MPF activity MPF peaks in M-phase Time ECB 18-10 M-phase Interphase Peak MPF induces M-phase 3. Surf clams and sea urchins: the abundance of “cyclin” proteins varies with the cell cycle Continuously label fertilized eggs with 35S-methionine Analyze incorporation into proteins by SDS-PAGE Cyclin A Cyclin B Ribonucleotide reductase (control) M-phase “Cyclin” abundance varies with cell cycle: MPF peaks in M-phase Cyclin B mRNA induces M-phase when injected into Xenopus oocytes MPF activity continuously synthesized… degraded at end of Mphase Interphase Cyclin synthesis Time ECB 18-6 M-phase Interphase Peak MPF induces M-phase Cyclin degraded Three models of the eukaryotic cell cycle wee1 cdc25 G2 cdc2 M cdc13 Cdc2 gene product is a master regulator of the G2-M transition MPF regulates entry into M-phase Bringing it all together Cyclin B mRNA (clam) induces Mphase in frog oocytes cdc13 encodes a yeast cyclin MPF consists of frog cdc2 homolog and cyclin B Abundance of “cyclins” in clam eggs varies with the cell cycle Cell cycle control: from models to molecules Inhibitory kinase CLB (cdc13) wee1 CLB cdc2 Inactive (weakly active) Active MPF (CDK1) P CLB CLB (cdc13) (cdc13) cdc2 P cdc2 P cdc2 CDK1 Inactive P (cdc13) Remove inhibitory phosphate P cdc25 Activating kinase Positive feedback ECB 18-11 and 18-12 “MPF” contains two components: cdc25 (inactive) cdc2 gene product = catalytic subunit of protein kinase cyclin B (CLB = cdc13): regulatory subunit activates kinase MPF = “Cyclin-dependent kinase (CDK1)” MPF (CDK1) activity is also regulated by phosphorylation wee 1 is inhibitory kinase cdc25 is activating phosphatase “Switching on” CDK1 (MPF) drives cell into M-phase Phosphorylate M-phase substrates Histones Lamins MAPs etc MPF triggers its own inactivation “anaphase promoting complex (APC)”; targets cyclin B for degradation Cyclin B accumulation activates MPF Metaphase (mid-M) High cyclin B MPF (CDK1) active Prophase (early-M) Activation of CDK1 by cyclin and cdc25 MPF activates APC CLB APC inactivates MPF by degrading cyclin B (cdc13) cdc2 P A cytoplasmic oscillator Accumulation of cyclin B APC APC Inactive CLB Active (cdc13) CLB cdc2 P (cdc13) Polyubiquitin Interphase APC is turned off cdc2 Telophase (late-M) Low cyclin B MPF inactive Cyclin B degraded by proteosome Anaphase Review: M-phase Interphase M-phase Interphase ECB 18-6 MPF activity MPF peaks in M-phase Cyclin synthesis Cyclin degraded Time Accumulation of cyclin B above threshold activates MPF (CDK1) and promotes entry into M-phase Activation of APC by MPF promotes cyclin destruction, MPF inactivation, and exit from M-phase Multiple CDKs regulate progression through the cell cycle Trigger M-phase M-phase cyclins (B) M-phase cyclin degraded… Active M-phase CDK (MPF) Done M-phase P M G1-CDKs; drive cells through G1 (won’t discuss) G2 M-phase CDK (CDK1) S-phase cyclins degraded… G1 S-phase CDKs S-phase cyclins and CDKs regulate DNA replication S-phase cyclins S P Active S-phase CDKs ECB 18-13 At least 6 different CDKs and multiple cyclins in mammals Trigger S-phase Degradation of Sphase cyclins promotes exit from S-phase into G2 S-Cdk regulates DNA replication Origin recognition complex protein scaffolding for assembly of other proteins Cdc6 increases in G1; binds ORC and induces binding of other proteins forming pre-replicative complex ECB 18-14 Origin is ready to fire Active S-Cdk 1- phosphorylates ORC causing origin to fire = replication 2-phosphorylates Cdc6 leading to ubiquitination and degradation Cdc6 not made until next G1 - prevents origin from double firing Completion of critical cellular processes is monitored at cell cycle “check points” ECB 18-17 Is the cell big enough? Is the environment favorable? Is DNA undamaged? Yes? Enter S phase Is DNA undamaged? Is DNA replicated? Is cell big enough? Yes? Enter M phase Have all chromosomes attached to spindle? Yes? Proceed to anaphase Of these, the G1/S checkpoint for damaged DNA is best understood The DNA damage checkpoint: p53 induced expression of an S-phase CDK inhibitor p53 (inactive) DNA damage activates p53 p53 (active) Active p53 acts as a transcription factor to turn on genes, including p21 RNA pol DNA p21 gene Transcription p21 protein inhibits G1/S phase CDKs, blocking entry into S-phase Translation p21 ECB 18-15 Cell arrests in G1 until damage repaired, or undergoes apoptosis (programmed cell death) P Active S-phase CDK P P21 binds and inactivates S-phase CDK If checkpoint is activated Exit cell cycle (temporary or permanent) neurons most plant cells Or undergo apoptosis (in a minute) Zones of division and growth in plant roots Arabidopsis thaliana Only a fraction of cells still actively dividing Zone of differentiation - cells cease growing and terminally differentiate Zone of cell elongation - growth but not division; Cells in G0 Meristem - zone of active cell division cells remain in cell cycle Regulation of each zone is not well understood in plants but involves hormones In animals: mitogens stimulate cell proliferation (block checkpoints) growth factors stimulate cell growth (stimulate biosynthesis, inhibit degradation) Apoptosis: A tale of tadpole tails and mouse paws what do they have in common? ECB figure 18-19 Tadpole tails are resorbed during metamorphosis ECB figure 18-18 Paws develop from “paddles” Both processes involve “programmed cell death (apoptosis)” ECB - “programmed cell death is a commonplace, normal, and benign event. It is the inappropriate proliferation and survival of cells that presents real dangers” Apoptosis is visibly distinct from necrosis ECB 18-20 Necrosis (cell death following injury) often results in lysis, spilling the contents into the surrounding space and causing inflamation During apoptosis (“programmed cell death”), cells remain intact and condense Corpses of apoptotic cells are often engulfed by their neighbors or specialized phagocytic cells Apoptosis is mediated by a “caspase cascade” Death protein Survival factor Caspase (inactive) Inactive Active “Caspases” are proteases; inactive precursors activated by proteolysis Presence of suicide signals and/or withdrawal of needed survival factor activates first caspase in cascade Initial caspase proteolytically activates downstream caspases …which activate additional caspases, and so on Activated caspases degrade nuclear and cytoplasmic proteins (lamins, cytoskeletal proteins, etc)… Activated endonucleases cut chromosomal DNA… ECB 18-21 Caspase cascade must be carefully regulated Bcl-2 family of proteins are death proteins Form pores in outer mitochondrial membrane releasing cytochrome c (respiratory chain) Cytochrome c binds adaptor and complex activates first procaspase