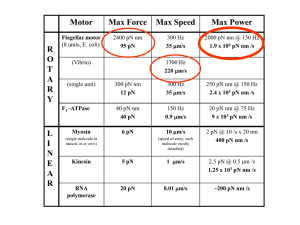
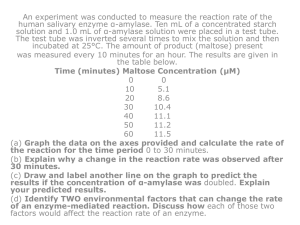
Presentation Slides
advertisement

GFPD FAMILY AND SCIENTIFIC CONFERENCE DEFINING HOW DIOSMETIN WORKS FOR PEX1-GLY483ASP AND PARTNERING WITH DRUG COMPANIES July 28th, 2013 Lincoln, Nebrasaka By Catherine Argyriou, Braverman laboratory McGill University Department of Human Genetics SUMMARY 1. The function of PEX1 a. The importance of PEX1 b. The PEX1-G843D allele c. Effect of PEX1 misfolding 2. Improving the function of PEX1-G843D a. Pharmacologic chaperone- diosmetin b. Proposed mechanism of action c. Designing the optimal drug d. Pharmaceutical company collaboration 3. The path forward a. Defining how diosmetin and other pharmacologic chaperones work b. Pharmacologic chaperones and other mutations THE FUNCTION OF PEX1 Crucial for peroxisome assembly Cleaves ATP to release energy which then allows the PEX1 protein to move Without this movement, PEX1 cannot pull PEX5 out of the membrane, therefore arresting peroxisome import THE MOVEMENT OF PEX1: AAA ATPASE Rouiller et al., 2002 THE PEX1-G843D ALLELE 20-30% of all alleles resulting in Zellweger Spectrum Disorder Presents milder phenotype No developmental defects, but multi-systemic effects due to progressive peroxisome dysfunction over time THE PEX1-G843D ALLELE Single amino acid substitution: Glycine Results in protein misfolding and degradation Hindered ability to cleave ATP Why do we see some function? Still able to complex with PEX6 at membrane Form a complex with reduced function Aspartate THE EFFECT OF PEX1 MISFOLDING Fujiki et al.,2012 IMPROVING THE FUNCTION OF PEX1G843D If protein is misfolded, can that be corrected? In vitro studies using patient cell lines indicate: PEX1-G843D may be amenable to proper folding Temperature (30ºC), chemical + pharmacologic chaperones Peroxisome function can improve IMPROVING THE FUNCTION OF PEX1G843D > 2000 compounds screened Flavonoids identified as effective in improving peroxisomal import Diosmetin Works in presence of PEX1-G843D protein, Does not work in PEX1 null cells, PEX6 null cells, or PEX 12 cells with a missense mutation Indicates some specificity, as expected with a pharmacologic chaperone DIOSMETIN: MECHANISM OF ACTION Flavonoids observed to bind at ATP binding pocket in other proteins Nguyen et al. 2006 Diosmetin may bind at the ATP binding site of PEX1G843D. DIOSMETIN: MECHANISM OF ACTION Flavonoids act on assembled membrane complex Gillian MacLean observed that diosmetin acts in PEX1+PEX6 complex at the peroxisomal membrane MacLean, 2013 DIOSMETIN: MECHANISM OF ACTION Our hypothesis: Following PEX1/PEX6 complex formation, diosmetin binds at the ATP binding sites of PEX1G843D and improves its conformation. Diosmetin binding is reversible. Diosmetin is released, and the properly-folded protein is now better able to bind ATP. GOAL: DESIGNING A FLAVONOID THAT BEST IMPROVES THE FUNCTION OF PEX1-G843D STEPS: Optimize drug structure of the for best fit into the PEX1-G843D complex Optimize drug structure to release a properly folded PEX1-G843D Ensure recovery of downstream peroxisome function Minimize toxicity Test in mouse model for clinical efficacy (Dr. Steinberg) OPTIMIZING DRUGS FOR PEX1-G843D: PARTNERING WITH PHARMACEUTICAL COMPANIES Diosmetin, scbt.com Pharmaceutical companies engineer many elaborations of a core compound Screen for best effect Minimal adverse effects OPTIMIZING DRUGS FOR PEX1-G843D: PARTNERING WITH PHARMACEUTICAL COMPANIES A not-for-profit organization that acts to bridge the gap between basic research and later stage drug development. OPTIMIZING DRUGS FOR PEX1-G843D: PARTNERING WITH PHARMACEUTICAL COMPANIES In order to design the best chemical substitutions, a company must know that the drug acts on the target protein. THE PATH FORWARD: DEFINING HOW DIOSMETIN AND OTHER PHARMACOLOGIC CHAPERONES WORK Does it bind PEX1-G843D? Require new tools Develop artificial system to model persoxisome import/export Develop best method to over-express tagged PEX1G843D and PEX6 Develop a bioinformatic approach to predict drug docking What is its effect on PEX1G843D? ATPase activity/ ATP hydrolysis/ ATP binding Establish kinetics of binding affinity THE PATH FORWARD: Chaperone therapy for other mutations Potential of chemical chaperones to benefit a broad ZSD patient population Via nonspecific interaction with misfolded proteins Develop general screening test to observe if lead compounds could effectively treat other ZSD mutations THE PATH FORWARD: Chaperone therapy for other mutations BacMam assay Baculovirus coupled with mammalian promoter Easy expression of GFP-PTS1 reporter in any patient cell line Easy screen for drug response Dolman et al., 2013 ACKNOWLEDGEMENTS Nancy Braverman Gillian MacLean Panteha Saberian & Sara Birjandian Steve Steinberg Joe Hacia NEOMED Institute Patients and Families for kindly providing cells for research Funding Organizations: