Chapter 1-15
The Big Ideas – E2 - I2
1. Evolution – the process of evolution drives the
diversity and unity of life.
2. Energy – biological systems utilize free energy and
molecular building blocks to grow, to reproduce,
and to maintain dynamic homeostasis.
3. Information – living systems store, retrieve,
transmit and respond to information essential to
life processes.
4. Interactions – biological systems interact and these
systems and their interactions possess complex
properties.
Carbon
•
•
•
•
•
•
Bonds covalently 4 times
Carbohydrates
Lipids
Proteins
Nucleic Acids
Isomers
Elements
Monomer
ID them
Uses/Roles
Examples
Structure/function relation
Dehydration vs. hydrolysis
Functional Groups
•
•
•
•
•
•
Hydroxyl
Amine
Carbonyl
Carboxyl
Sulfhydryl
Phosphate
Sulfhydryl
Cells
•
•
•
•
•
Microscopes
Cell fractionation
Cell Size
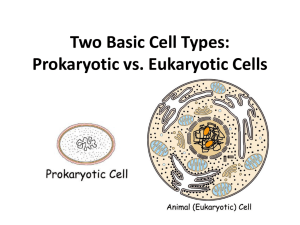
Prokaryotic vs. Eukaryotic
Animal vs. Plant
Surface Area and Volume Math
Question
• What is the SA/V for this cell? Round your
answer to the nearest hundredths.
Answer
SA = 4πr2
= 4(3.14) 52
= 314
Volume of a sphere= 4/3 π r3
= 4/3 (3.14)53
=523.33
SA/V=314/523.33
=.60
Endomembrane System
•
•
•
•
•
•
Rough ER
Smooth ER
Golgi Apparatus
Nucleus
Nuclear envelope
Nucleolus
Organelles
•
•
•
•
•
•
Ribosome
Lysosome
Peroxisome
Vacuole
Chloroplast
Mitochondria
Cytoskeleton
•
•
•
•
•
•
•
•
Centrosomes
Centrioles
Cilia
Flagella
Basal body
Microtubules
Intermediate filaments
Microfilaments
Cell Surface
• Cell wall
• Extracellular matrix
• Intercellular junctions
– Plasmodesmata/gap junctions
– Tight junctions
– desmosomes
Gap
Desmosomes
Tight
During an investigation of a freshwater lake, an AP Biology student discovers a previously
unknown microscopic organism. Further study shows that the unicellular organism is eukaryotic.
(a) Identify FOUR organelles that should be present in the eukaryotic organism and describe the
function of each organelle.
(b) Prokaryotic cells lack membrane-bound organelles found in eukaryotes. However,
prokaryotes must perform many of the same functions as eukaryotes. For THREE of the
organelles identified in part (a), explain how prokaryotic cells carry out the associated functions.
(c) According to the endosymbiotic theory, some organelles are believed to have evolved
through a symbiotic relationship between eukaryotic and prokaryotic cells. Describe THREE
observations that support the endosymbiotic theory.
Membrane structure and Function
• Fluid mosaic model
– Phospholipids
– Glycolipid
– Glycoprotein
– Intergral proteins
– Peripheral proteins
– Transport proteins
Diffusion
• Definition
• Factors that effect diffusion
• Problems:
– The molar concentration of a
sugar solution in an open
beaker has been determined
to be 0.3M. Calculate the
solute potential at 27 oC.
Round your answer to the
nearest tenths.
Solute potential= –iCRT
i = The number of particles the
molecule will make in water; for
NaCl this would be 2; for
sucrose or glucose, this number
is 1
C = Molar concentration (from
your experimental data)
R = Pressure constant = 0.0831
liter bar/mole K
T = Temperature in degrees
Kelvin = 273 + °C of solution
Answer
• Solute potential= –iCRT
-i= 1
C= 0.3
R = Pressure constant = 0.0831
T= 27 +273=300K
Solute concentration= -7.5
Osmosis
•
•
•
•
•
•
Definition
Hypotonic, Hypertonic, Isotonic
Water potential
Aquaporins
Osmoregulation
If ΨP = 0.3 MPa and ΨS = -0.45 MPa, the resulting Ψ is
–
–
–
–
a.
b.
c.
d.
+0.75 MPa.
-0.75 MPa.
-0.15 MPa.
+0.15 MPa.
Other types of transport
•
•
•
•
•
•
•
Facilitated diffusion
Active transport
Endocytosis
Exocytosis
Pinocytosis
Phagocytosis
Receptor
mediated
endocytosis
Energy and the Cell
•
•
•
•
•
Metabolism
1st and 2nd law of thermodynamics
Kinetic vs. Potential energy
Endergonic vs. Exergonic
Energy coupling
– ATP
– Phosphorylation
Enzymes
•
•
•
•
Activation energy
Active site
Induced fit
Things that effect the functioning of an enzyme
–
–
–
–
Temperature
pH
Concentration
Competitive or
noncompetitive
inhibition
– Cofactors
• Allosteric regulation
• Cooperativity
Cellular Respiration
•
•
•
•
•
•
C6H12O6 + 6O2 ---> 6CO2 + 6H2O + ATP
Redox reactions
Glycolysis
Krebs
Electron transport
Anaerobic respiration (fermentation)
Energy Harvest Phase
Order of electron carriers:
FMN Fe-S Q Cyt b Fe-S Cyt c1 Cyt c Cyt a Cyt a3
Microbes
produce acetone
or methanol
An agricultural biologist was evaluating two newly developed varieties of wheat as potential
crops. In an experiment, seedlings were germinated on moist paper towels at 20ºC for 48 hours.
Oxygen consumption of the two-day-old seedlings was measured at different temperatures. The
data are shown in the graph below.
(a) Calculate the rates of oxygen consumption in mL/min for each variety of wheat at 7°C and at
17°C. Show your work (including your setup and calculation).
(b) Explain the relationship between metabolism and oxygen consumption. Discuss the effect of
temperature on metabolism for each variety of seedlings.
(c) In a second experiment, variety A seedlings at both temperatures were treated with a
chemical that prevents NADH from being oxidized to NAD+. Predict the most likely effect of the
chemical on metabolism and oxygen consumption of the treated seedlings. Explain your
prediction.
The element carbon is contained in all organic compounds.
(a) Discuss the role of photosynthesis and cellular respiration in carbon cycling in the biosphere.
(b) For THREE of the following, predict and explain the effect on the carbon cycle if:
-- decomposers were absent
-- deforestation occurred
-- volcanic dust accumulated in the atmosphere
-- the average ocean temperature increased
(c) Explain how increased CO2 in the atmosphere results in greater acidification of oceans and
describe the effect on marine organisms. Include in your discussion TWO examples of how
human activity can increase atmospheric CO2.
Photosynthesis
• 6 CO2 + 12 H2O + lt nrg C6H12O6 + 6 O2 + 6 H2O
• Light reaction
– Cyclic electron vs.
noncyclic electron flow
• Dark reaction
– Carbon fixation
– Reduction
– Regeneration
C3/Photorespiration
• When Rubisco accepts O2
instead of CO2 as the
substrate.
• Generates no ATP.
• Decreases Ps output by as
much as 50%.
C4
• Uses a different enzyme to
initially capture CO2
• Separates CO2 capture
from carbon fixation
• Still uses C3 Ps to make
sugar, but only does so in
the bundle sheath cells.
CAM
• Open stomata at night
to take in CO2.
• The CO2 is stored as a
C4 acid.
• During the day, the acid
is broken down and CO2
is fixed into sugar.
• Still uses C3 Ps to
make sugar.
• Slow growth
Cell Communication
• Reception
– Direct signaling
– Local signaling
– Long distance
• Transduction
• Response
Reception
•
•
•
•
•
•
Signal molecules
Receptor molecules
G-protein coupled
Tyrosine-kinase
Ion channels
Intracellular
Signal Transduction
•
•
•
•
Amplification
Protein Kinase
Protein phosphatases
Secondary messengers
Responses
• Rearrange cytoskeleton
• Transcription
Cell Cycle
• Cell Cycle
– G1 , S , G2
• Checkpoints
– G1 , G2 , M phase
• Mitosis
– Prophase
– Metaphase
– Anaphase
– Telophase/Cytokinesis
Meiosis
•
•
•
•
•
•
•
Diploid Haploid
2 divisions
Testicular/Ovarian cell to make sperm/egg
Independent assortment
Crossing over
Increases variety
Random fertilization
Mutations
1. The cell cycle is fundamental to the reproduction of eukaryotic cells.
(a) Describe the phases of the cell cycle.
(b) Explain the role of THREE of the following in mitosis or cytokinesis.
Kinetochores
Microtubules
Motor proteins
Actin filaments
(c) Describe how the cell cycle is regulated and discuss ONE consequence of abnormal
regulation
Mendel Practice
• Two heterozygotes produce 345 offspring
– What is your expected phenotypic ratio? 3:1
– How many individuals are expected to have the
dominant phenotype? 259
– How many individuals are expected to have the
recessive phenotype? 86
• In this genetic cross Aa x aa there are 714
offspring
– How many individuals are expected to have the
dominant phenotype? 357
– How many individuals are expected to have the
recessive phenotype? 357
Mendel Practice
• In a dihybrid cross between two heterozygotes, if
you have 360 offspring, what are your expected
values?
– Both dominant phenotypes 9/16 = .56 = 56% = 202
– One dominant; one recessive 3/16 = .19 = 19% = 68
– One recessive; one dominant 3/16 = .19 = 19% = 68
– Both recessive phenotypes 1/16 = .06 = 6% = 22
Exceptions to Mendel
•
•
•
•
•
•
•
Incomplete dominance
Codominance
Sex-linked traits
Multiple alleles
Pleiotropy
Epistasis
Polygenic inheritance
Chapter 15 highlights
• Sex linked traits
– examples
– Seen more in males…why?
• X-inactivation
• Gene mapping using recombination frequency
• Chromosomal Mutations
– Nondisjunction, deletion, duplication, inversion,
translocation
Questions Part 1 Review Booklet
• How do the unique chemical and physical
properties of water make life on earth
possible?
– High specific Heat
– Adhesion
– Cohesion
– Polarity
• What is the role of carbon in the diversity of
life?
• How do cells synthesize and breakdown
macromolecules?
• How do structures of biological molecules
account for their function (carbs, proteins,
lipids, and DNA)
• What are the similarities and differences
between prokaryotic and eukaryotic cells?
• What are the evolutionary relationships
between prokaryotic and eukaryotic cells?
• How does compartmentalization organize a
cell’s functions?
• How are the structures of the various
subcellular organelles related to their
function?
• How do organelles function together in
cellular processes?
• What is the current model of molecular
architecture of membranes?
• How do variations in this structure account for
functional differences among membranes?
• How does the structure of membranes
provide for transport and recognition?
• What are various mechanisms by which
substances can cross the membrane?
• In osmosis and diffusion lab, how was osmosis
measure in both living (cells/potatoes) and
artificial (dialysis tubing)?
• What was the independent variable in the dialysis
bag part of the lab?
– Dependent variable?
– Control?
– Controlled variables?
• What was the independent variable in the
potato part of the lab?
– Dependent variable?
– Control?
– Controlled variables?
Concept Map
Atom, compound, carbohydrate,
lipid, protein, nucleic acid,
organelles, nucleus,
mitochondria, cell membrane,
golgi apparatus, ER, prokaryotic
cell, eukaryotic cell
Questions Part 2 Review Booklet
• How do the laws of thermodynamics relate to
the biochemical processes that provide energy
to living systems?
• How do enzymes regulate the rate of chemical
reactions?
• How does the specificity of an enzyme depend
of its structure?
• How is the activity of an enzyme regulated?
• How does the cell cycle assure genetic
continuity?
• How does mitosis allow for the even
distribution of genetic information to new
cells?
• What are the mechanisms of cytokinesis?
• How is the cell cycle regulated?
• How can aberrations in the cell cycle lead to
tumor formation?
• Why is meiosis important in heredity?
• How is meiosis related to gametogenesis?
• What are the similarities and differences
between gametogenesis and animals and
plants?
• What is the role of ATP in coupling the cell’s
anabolic and catabolic processes?
• How does chemiosmosis function in
bioenergetics?
• How are organic molecules broken down by
catabolic pathways?
• What is the role of oxygen in energy-yielding
pathways?
• How do cells generate ATP in the absence of
oxygen?
• How does photosynthesis convert light energy
into chemical energy?
• How are the chemical products of the lighttrapping reactions coupled to the synthesis of
carbohydrates?
• What kinds of photosynthetic adaptations have
evolved in response to different environmental
conditions?
• What interactions exist between
photosynthesis and cellular respiration?
• How was photosynthetic rate measured in the
photosynthesis lab?
• What was the independent variable in the
photosynthesis lab?
– Dependent variable?
– Control?
– Controlled variables?
• How was respiration rate measured in the
respiration lab (pea lab)?
• What was the independent variable in the
lab?
– Dependent variable?
– Control?
– Controlled variables?
Concept map
Cell cycle, interphase, growth,
DNA replication, mitosis, meiosis,
homologous chromosomes,
separations of chromosomes,
cancer, checkpoints, regulatory
proteins.
Concept Map
Unicellular, multicellular, local regulators,
long distance regulation, contact, receptor,
signal transduction, enzyme cascade,
response