Biology of post-transplant lymphoproliferative disorders and other
advertisement

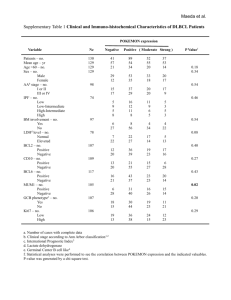
Imperial College London A comparison of the frequency of common lymphomaassociated gene rearrangements among B-Post transplant lymphoproliferative disorders (B-PTLD), B-cell HIVlymphomas and diffuse large B-cell lymphoma in immune competent patients (iDLBCL). Hazem AH Ibrahim, Michael J Neat, Mufaddal Moonim, Amen Furrat, Lia Menasce, Sabine Pomplun, Margaret Burke, Donald Macdonald, Ed Kanfer, Mark Bower, Paul Fields, Nicola Foot, Alistair Reid and Kikkeri N Naresh. Department of Histopathology & Cytopathology, Hammersmith Hospital Background Post-transplant lymphoproliferative disorders (PTLD) • Early lesions • Polymorphic PTLD • Monomorphic PTLD • Classical Hodgkin lymphoma-type PTLD Monomorphic PTLD • B-cell neoplasms DLBCL Burkitt lymphoma Plasmacytoma / plasma cell myeloma Plasmacytoma-like Others • T-cell neoplasms (~15%) Peripheral T-cell lymphoma, NOS Hepatosplenic T-cell lymphoma Others HIV-LPDs • • • • • • Burkitt lymphoma. Diffuse large B-cell lymphoma (DLBCL). Primary effusion lymphoma (PEL). Plasmablastic lymphoma. HHV-8-associated LPDs in patients of MCD. Polymorphic lymphoid proliferations resembling PTLDs. Aetiology • Viruses: 1) EBV 2) HHV-8 • Genetic changes (translocation, Mutation,......) • Antigen stimulation 20-40% of iDLBCL and HIV-related DLBCLs harbour BCL6 rearrangement that are very rarely seen in PTLDs. Post-transplant Burkitt lymphomas (PT-BL), similar to HIV-BL and iBL, display chromosomal breaks at 8q24 involving the c-MYC oncogene. Very few reports investigated chromosomal translocations among PTLDs Question ? Do common lymphoma-associated gene rearrangements differ among B-PTLDs, B-cell HIVlymphomas and iDLBCL? Materials & Methods Tissue microarray Cases collected • 64 B-PTLD TMA • 41 HIV-BCL Serial sections • 139 iDLBCL IHC ISH H&E FISH Genes investigated BCL2, BCL3, BCL6, c-MYC, PAX5, MALT1 and IGH Results Percentage involvement of rearrangements of different genes among DLBCLs in different settings BCL6 23% BCL6 BCL2 c-MYC BCL3 IGH only BCL2 11% None 53% Double hit 5% IGH only 2% c-MYC 4% BCL3 2% Double hit None iDLBCL (139 cases) c-MYC 8% c-MYC 30% c-MYC c-MYC double hits None None None 62% double hits 8% None 92% PTLD-DLBCL(24 cases) HIV-DLBCL(39cases) •BCL2 and BCL6 rearrangements were predominantly restricted to GC and AGC/non-GC subtypes respectively. •8% PT-DLBCLs and 30% HIV-DLBCLs showed cMYC rearrangement. •PT-DLBCLs and HIV-DLBCLs lacked BCL2 and BCL6 rearrangements. •Seven iDLBCLs (5%) and 2 HIV-DLBCLs (8%) had rearrangements of two oncogenes. •Among Burkitt lymphoma (BL), 2/2 PT-BL and 12/13 (92%) HIV-BL had c-MYC rearrangement. •Among plasmablastic lymphoma (PL), 2/6 (33%) PT-PL and 1/2 HIV-PL had c-MYC rearrangement. Rearrangements of different genes among iDLBCL subsets Negative AGC/non GC (MUM1-pos) IGH only Double hit c-MYC BCL3 BCL6 BCL2 Negative GC (MUM-neg) IGH only Double hit c-MYC BCL3 BCL6 BCL2 0% 10% 20% 30% 40% 50% 60% EBV association •EBV-association was noted in 6%, 67% and 54% of iDLBCLs, PT-DLBCLs and HIV-DLBCLs respectively. •100% PT-BL and 63% HIV-BL had EBV-association. •83% PT-PL and 100% HIV-PL had EBV-association. Correlation of rearrangements of c-MYC, BCL2 and BCL6 genes among the EBV-positive cases •None of the cases with either BCL2 or BCL6 rearrangement showed EBV-association (p=0.031 & p<0.001 respectively). • No significant correlation between EBVassociation and c-MYC or IGH rearrangement. EBER Dual-colour break-apart probes C-MYC IGH Monomorphic PTLD, plasmablastic lymphoma Summary and Conclusion 1- Gene rearrangements • Gene rearrangement, apart from c-MYC-IGH (characteristically seen in BL and PL), appear to be very rare among both HIV-BCL and BPTLD. • HIV-DLBCL is more frequently associated with c-MYC rearrangement than iDLBCL. • BCL6 rearrangement is frequently seen in iDLBCL of AGC and non-GC subtypes. • None of the cases with either BCL2 or BCL6 rearrangement showed EBV-association. 2- Alternate pathogenetic pathways in immune deficiency LPDs • Other (cyto)genetic abnormalities that are that are not conventionally associated primary abnormalities in lymphomas • Aberrant somatic hypermutation of critical genes. • Aberrant hypermethylation of critical genes. References 1. Jaffe ES, Harris NL, Stein H et al: World Health Organization Classification of Tumour: Pathology and Genetics, Tumours of Haematopoietic and Lymphoid Tissues. IARC Press: Lyon, 2008. 2. Vega F, Medeiros LJ: Chromosomal translocations involved in non-Hodgkin lymphomas. Arch Pathol Lab Med 127:1148-1160, 2003. 3. Tibiletti MG, Martin V, Bernasconi B et al: BCL2, BCL6, MYC, MALT 1, and BCL10 rearrangements in nodal diffuse large B-cell lymphomas: a multicenter evaluation of a new set of fluorescent in situ hybridization probes and correlation with clinical outcome. Hum Pathol 40:645-652, 2009. 4. Vakiani E, Nandula SV, Subramaniyam S et al: Cytogenetic analysis of B-cell posttransplant lymphoproliferations validates the World Health Organization classification and suggests inclusion of florid follicular hyperplasia as a precursor lesion. Hum Pathol 38:315-325, 2007. 5. Gaidano G, Lo CF, Ye BH et al: Rearrangements of the BCL-6 gene in acquired immunodeficiency syndrome-associated non-Hodgkin's lymphoma: association with diffuse large-cell subtype. Blood 84:397-402, 1994 6. Windebank K, Walwyn T, Kirk R et al: Post cardiac transplantation lymphoproliferative disorder presenting as t(8;14) Burkitt leukaemia/lymphoma treated with low intensity chemotherapy and rituximab. Pediatr Blood Cancer 53:392-396, 2009. 7. Capello D, Rossi D, Gaidano G: Post-transplant lymphoproliferative disorders: molecular basis of disease histogenesis and pathogenesis. Hematol Oncol 23:61-67, 2005 Acknowledgements The Egyptian Government • • • • • Prof. Kikkeri Naresh Prof. Gordon Stamp. Dr Roberto Dina Mahrokh Nohdani. Donna Homcastle, Pritesh Trivedi, Tyler Lloyd & Kay Elderfield. • William Mathieson • John Brennan and David Peston. • Prof. Letizia Foroni, Alistair Raid, and Jamshid Sorouri. All members of the Department of Histopathology, of Hammersmith Hospital. Thank you