Nuclear Chemistry
advertisement

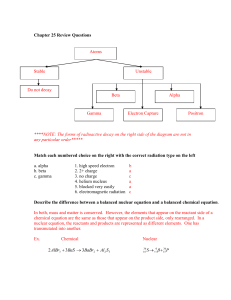
Nuclear Chemistry The Band of Stability Nuclear Stability • The Band of stability allows us to analyze the proton to neutron ratio of various nuclides. • A nucleus with a proton to neutron ratio that is 1:1 is considered to be stable. Anything that varies that can lead to an unstable nucleus. • At atomic number 20, more neutrons are needed than protons to keep the nucleus stable. • At atomic number 82 virtually no nucleus is stable. Particles in Nuclear Equations • Sub-atomic particles in nuclear equations: (A proton is also sometimes represented as .) • Alpha particles (symbolized α or ) are identical to helium-4 nuclei, so the helium chemical symbol may also be used to represent an alpha particle. • Beta particles (symbolized β, β–, or ) are highspeed electrons. (The emission of electrons from the nucleus may seem strange, but they result from a reaction in the nucleus) • Gamma particles (symbolized γ, or ) are very highenergy photons. Alpha Decay • The emission of alpha particles. • In nuclear equations the sum of the mass number (symbolized by A) on both sides of the arrow must be equal. • The sum of the atomic numbers (symbolized by Z) must also be equal on both sides. Remember that only the atomic number identifies the element. Beta Decay • The emission of beta particles (high speed electrons). Positron (β+) emission • Involves the emission of a β+ particle from the nucleus. A key idea of modern physics is that most fundamental particles have corresponding antiparticles with the same mass but opposite charge. (The neutrino and antineutrino are an example.) • The positron is the antiparticle of the electron. Positron emission occurs through a process in which a proton in the nucleus is converted into a neutron, and a positron is expelled. In terms of the effect on A and Z, positron emission has the opposite effect of β− decay: the daughter has the same A but Z is one lower (one fewer proton) than the parent. Thus, an atom of the element with the next lower atomic number forms. Carbon-11, a synthetic radioisotope, decays to a stable boron isotope through β+ Electron (e−) capture (EC) • Occurs when the nucleus interacts with an electron in an orbital from a low atomic energy level. The net effect is that a proton is transformed into a neutron: • (We use the symbol “e” to distinguish an orbital electron from a beta particle, β.) The orbital vacancy is quickly filled by an electron that moves down from a higher energy level, and that process continues through still higher energy levels, with x-ray photons and neutrinos carrying off the energy difference in each step. Radioactive iron forms stable manganese through electron capture: • Even though the processes are different, electron capture has the same net effect as positron emission : Z lower by 1, A unchanged. Gamma (γ) emission • Involves the radiation of high-energy γ photons from an excited nucleus. Just as an atom in an excited electronic state reduces its energy by emitting γ photons, usually in the UV and visible ranges, a nucleus in an excited state lowers its energy by emitting photons, which are of much higher energy (much shorter wavelength) than UV photons. Many nuclear processes leave the nucleus in an excited state, so γ emission accompanies many other (but mostly β ) types of decay. Several γ photons (also called γ rays) of different energies can be emitted from an excited nucleus as it returns to the ground state. Some of Marie Curie’s experiments involved the release of γ rays, such as • Gamma emission is common subsequent to β− decay, as in the following: Nuclear Transmutation • The formation of different elements by the bombardment of other particles. For example the alpha bombardment of nitrogen-14: Common Transmutations Half-lives • A half-life (t1/2) is the time it takes for a radioactive sample to decay into half the original amount. Nuclear Fission • The splitting of large unstable nuclei. • A neutron bombarding a 235U nucleus results in an extremely unstable 236U nucleus, which becomes distorted in the act of splitting. In this case, which shows one of many possible splitting patterns, the products are 92Kr and 141Ba. Three neutrons and a great deal of energy are released also. Fission A chain reaction of uranium-235. Nuclear Fusion • The fusion of light nuclei. • These reactions usually involve isotopes of hydrogen, helium, and lithium. • These reactions occur in the sun. They are being studied in order to harness the energy produced as a result of a fusion reaction. The tokamak design for magnetic containment of a fusion plasma. • The donut-shaped chamber of the tokamak (photo, top; schematic, bottom) contains the plasma within a helical magnetic field. Radiation Exposure • Radiation exposure in relation to tissue damage is measured in roentgen equivalent for man (rem). The following table reflects what would occur when there is whole body radiation exposure. Common Uses of Radioactive Isotopes Practice Set 1: Nuclear Equations • • • • • • • • • • (1) Alpha decay of uranium-234 (2) Electron capture by neptunium-232 (3) Positron emission by nitrogen-12 (4) β− decay of sodium-26 (5) β− decay of francium-223 (6) Alpha decay of bismuth-212 (7) β− emission by magnesium-27 (8) β+ emission by (9) Electron capture by (10)β− decay of silicon-32 Practice Set 2: Nuclear Equations • • • • • • • • • • (11) Alpha decay of polonium-218 (12)Electron capture by (13)Formation of titanium-48 through positron emission (14)Formation of silver-107 through electron capture (15)Formation of polonium-206 through α decay (16)Formation of β− through decay (17)Formation of β− through decay (18)Formation of bismuth-203 through α decay (19)Formation of 186Ir through electron capture (20)Formation of francium-221 through α decay