Note: For nuclear transmutation reactions, the following symbolism
advertisement
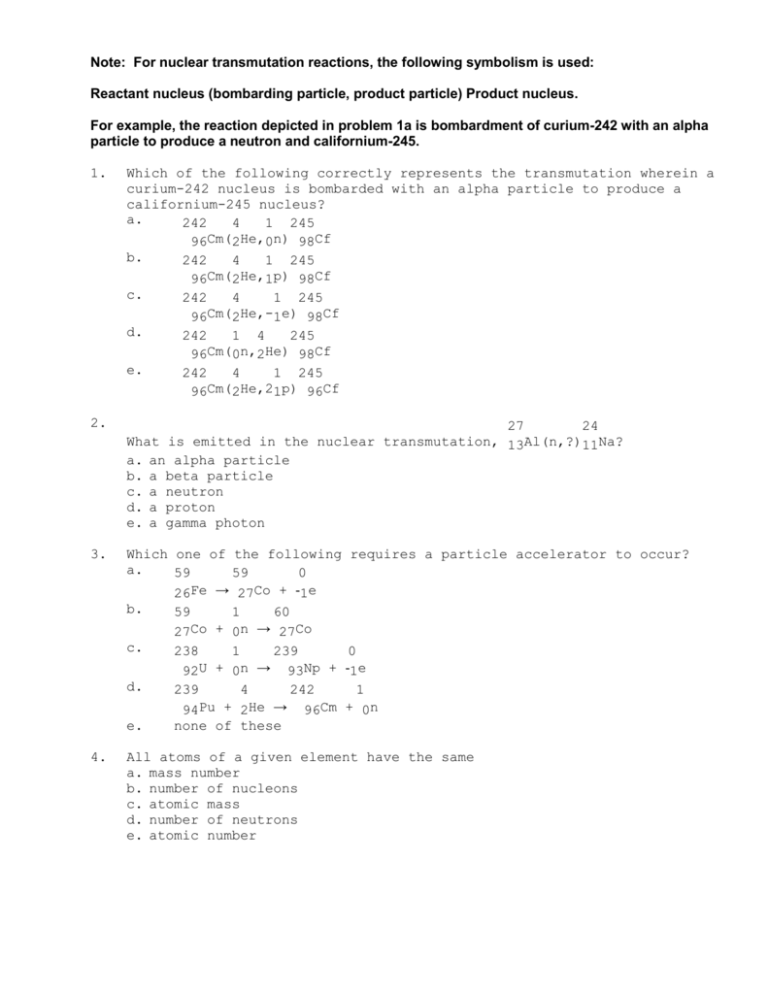
Note: For nuclear transmutation reactions, the following symbolism is used: Reactant nucleus (bombarding particle, product particle) Product nucleus. For example, the reaction depicted in problem 1a is bombardment of curium-242 with an alpha particle to produce a neutron and californium-245. 1. Which of the following correctly represents the transmutation wherein a curium-242 nucleus is bombarded with an alpha particle to produce a californium-245 nucleus? a. 242 4 1 245 96Cm(2He,0n) 98Cf b. 242 4 1 245 Cm( He, 96 2 1p) 98Cf c. 242 4 1 245 96Cm(2He,-1e) 98Cf d. 242 1 4 245 Cm( n, He) 96 0 2 98Cf e. 242 4 1 245 96Cm(2He,21p) 96Cf 2. 27 24 What is emitted in the nuclear transmutation, 13Al(n,?)11Na? a. an alpha particle b. a beta particle c. a neutron d. a proton e. a gamma photon 3. Which one of the following requires a particle accelerator to occur? a. 59 59 0 Fe → Co + 26 27 1e b. 59 1 60 27Co + 0n → 27Co c. 238 1 239 0 U + n → Np + 92 0 93 1e d. 239 4 242 1 Pu + He → Cm + 94 2 96 0n e. none of these 4. All atoms of a given element have the same a. mass number b. number of nucleons c. atomic mass d. number of neutrons e. atomic number 5. 41Ca decays by electron capture. The product of this reaction undergoes alpha decay. What is the product of this second decay reaction? a. Ti b. Ca c. Ar d. Cl e. Sc 6. What is the largest number of protons that can exist in a nucleus and still be stable? a. 206 b. 50 c. 92 d. 83 e. 84 7. This reaction is an example of ___________. a. b. c. d. e. 41 41 20Ca → 19K + __________ alpha decay beta decay positron decay electron capture gamma emission 8. Of a. b. c. d. e. the following processes, which one changes the atomic number? alpha emission beta emission electron capture positron emission all of these processes change the atomic numbers 9. By a. b. c. d. e. what process does thorium-230 decay to radium-226? gamma emission alpha emission beta emission electron capture positron emission 10. What is the missing product from this reaction? 32 32 P → 15 16S + _______ a. 4 2He b. 0 -1e c. d. 0 1e 0 1P e. 11. Alpha-decay produces a new nucleus whose ___________________ than those respectively of the original nucleus. a. atomic number is 2 less and mass number is 2 less b. atomic number is 1 less and mass number is 2 less c. atomic number is 2 less and mass number is 4 less d. atomic number is 2 more and mass number is 4 more e. atomic number is 2 more and mass number is 2 less 12. Stable nuclei with low atomic numbers, up to 20, have a neutron to proton ratio of approximately ___. 13. N0 t, to a. b. c. d. e. and Nt represent the amount of a radionuclide initially and at time respectively. After a half-life has passed (t=t1/2), the ratio of N0 Nt is equal to two is equal to one half depends on the half-life of the radionuclide depends on the rate constant of the decay process depends on the type of radioactive decay 14. Consider the following data for a particular radionuclide (graph the data!): time 0 min 3 6 9 12 Nt 1.23 g 1.15 1.08 1.01 0.940 What is the rate constant (in min-1) radionuclide? a. 44.64 b. 30.9 c. 0.0242 d. 0.0324 e. 0.0224 for the decay of this 15. What order process is radioactive decay? a. zeroth b. first c. second d. third e. fourth 16. The beta decay of cesium-137 has a half-life of 30 years. How many years must pass to reduce a 25 mg sample of cesium 137 to 8.7 mg? a. 46 b. 32 c. 3.2 d. 50 e. 52 17. 210Pb has a half-life of 22.3 years and decays to produce 206Hg. If you start with 7.50 g of 210Pb, how many grams of 206Hg will you have after 17.5 years? a. 4.35 b. 3.15 c. 3.09 d. 0.0600 e. 1.71 18. The carbon-14 dating method can be used to determine the age of a a. flint arrowhead b. papyrus scroll c. stone axe head d. clay pot e. rock 19. Which one of the following processes results in an increase in the atomic number? a. gamma emission b. positron emission c. beta emission d. alpha emission e. corrosion 20. Plutonium-239 undergoes alpha decay and has a half-life of 24,000 years. How much will be left of a 1.000-mg sample of plutonium after 20000 years? a. 0.561 b. 0.833 c. 1.2 d. 0.422 e. 0.578 21. What is a tokamak? a. a device that uses strong magnetic fields to contain and heat nuclei with the goal of inducing sustained fusion b. a linear particle accelerator c. a variation on a cyclotron d. a laser heating device e. a fission bomb 22. What is the binding energy (in J) of a nucleus with a mass defect of 0.2 amu? a. 2.990 X 10-14 b. 2.990 X 10-19 c. 2.990 X 10-11 d. 2.990 X 10-8 e. 1.8 X 1013 23. The mass of a proton is 1.00728 amu and that of a neutron is 1.00867 60 amu. What is the binding energy (in J) of a 28Ni nucleus? (The mass of a nickel-60 nucleus is 59.9308 amu.) a. 2.759 X 10-16 b. 9.211 X 10-28 c. 4.964 X 10-13 d. 2.773 X 10-19 e. 8.230 X 10-11 24. The mass of a proton is 1.00728 amu and that of a neutron is 1.00867 60 amu. What is the binding energy per nucleon (in J) of a 28Ni nucleus? (The mass of a nickel-60 nucleus is 59.9308 amu.) a. 2.501 X 10-12 b. 3.046 X 10-12 c. 1.372 X 10-12 d. 9.451 X 10-13 e. 7.028 X 10-14 25. When two atoms of 2H are fused to form one atom of 4He, the total energy evolved is 3.83 X 10-12 J. What is the total change in mass (in Kg) for this reaction? (C = 3.00 X 108 m/s) a. 1.28 X 10-17 b. 4.26 X 10-26 c. 3.45 X 108 d. 1.15 e. 4.26 X 10-29 26. How many neutrons are emitted when a californium-249 nucleus (Z=98) 257 is bombarded with a carbon-12 nucleus to produce a 104Rf nucleus? a. one b. three c. two d. four e. zero 27. The decay of a radionuclide with a half-life of 4.3 X 105 years has a rate constant (in yr-1) equal to a. 6.2 X 105 b. 1.6 X 10-6 c. 2.3 X 10-6 d. 2.8 X 103 e. 5.9 X 10-8 28. When ionizing radiation enters the body, what is the predominant free radical produced (see part D of radioactivity experiment)? a. H b. H3O c. protein d. OH e. H2O 29. What exposure level to radiation is fatal to most humans? a. 100 rem b. 200 rem c. 600 rem d. 300 rem e. 1000 rem 30. What is the typical percent of uranium-235 in the enriched UO2 pellets used in nuclear reactors? a. 0.7 b. 1 c. 3 d. 5 e. 14 31. On average, __________ neutrons are produced by every fission of uranium-235. a. 4 b. 3.5 c. 1 d. 2.4 e. 2 32. What is the purpose of the moderator in a nuclear reactor? 33. What drives the turbine in a nuclear power plant? a. the moderator b. steam c. the control rods d. the primary coolant e. UF6 gas 34. What type of reaction is known as a thermonuclear reaction? a. fission b. fusion c. transmutation d. beta emission e. neutron emission 35. The main scientific difficulty in achieving a controlled fusion process is the a. enormous repulsion between nuclei being fused b. enormous repulsion between the electrons of atoms being fused c. very large number of positrons emitted d. very large number of x-rays emitted e. very large number of gamma rays emitted 36. The relative biological effectiveness (RBE) values of beta rays, gamma rays, and alpha rays are, respectively 37. What exposure level to radiation is fatal to most humans? a. 100 rem b. 200 rem c. 600 rem d. 300 rem e. 1000 rem 38. The noble gas thought to be significantly carcinogenic due to its radioactive decay and that of its decay products is a. helium b. xenon c. argon d. radon e. neon 39. The curie is a measure of the a. number of disintegrations per second of a radioactive substance. b. total energy absorbed by an object exposed to a radioactive source. c. lethal threshold for radiation exposure. d. number of alpha particles emitted by exactly one gram of a radioactive substance. e. none of these is correct. 40. Which one of the following forms of radiation can penetrate the deepest into body tissue? a. alpha b. beta c. gamma d. positron e. proton 41. Which one of the following can be done to shorten the half life of the radioactive decay of uranium-238? a. freeze it b. heat it c. convert it to UF6 d. oxidize it to the +2 oxidation state e. none of these 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 11. 12. 13. 14. 15. 16. 17. 18. 19. 20. 21. 22. 23. 24. 25. 26. 27. 28. 29. 30. 31. 32. 33. 34. 35. 36. 37. 38. 39. 40. 41. a a d e d d c e b b c 1 a e b a c b c a a c e c e d b d c c d to slow the neutrons so that they can be captured by the fissile uranium-235 and cause subsequent fission reactions b b a 1, 1, 10 c d a c e