sch4u-quantumtheory
advertisement

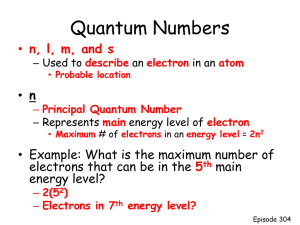
Rise of the Quantum Theory • Light – particles or waves? Greeks answer: particles • 17th century, Christian Huygens, proposed light can be best described as a wave; Isaac Newton vehemently opposed • Mid-19th century, James Maxwell proposed that light is an electromagnetic wave consisting of magnetic and electric fields that can exert forces on an object (Classical Theory of light) The Wave Nature of Light Electromagnetic waves originate from the movement of electric charges The movement produces fluctuations in electric and magnetic fields Characterizing Waves Electromagnetic radiation is characterized by its wavelength, frequency, and amplitude Wavelength (l) is the distance between any two identical points in consecutive cycles Characterizing Waves Frequency of a wave is the number of cycles of the wave that pass through a point in a unit of time Amplitude of a wave is its height: the distance from a line of no disturbance through the center of the wave peak The Electromagnetic Spectrum The electromagnetic spectrum is largely invisible to the eye The Electromagnetic Spectrum • We can feel some radiation through other senses (infrared) • Sunburned skin is a sign of too much ultraviolet radiation • Materials vary in their ability to absorb or transmit different wavelengths – Our bodies absorb visible light, but transmit most X rays – Window glass transmits visible light, but absorbs ultraviolet radiation Bright Line & Dark Line Spectra • Robert Bunsen & Gustav Kirchhoff invented the spectroscope (1859) • They found that energized gases emit coloured light • Different types of gases emit different colours of light • Light from energized elements (gaseous form) produced specific bands of colour => bright line or emission line spectrum • What is a dark line or absorption spectrum? The Continuous Spectrum l ~ 650 nm l ~ 575 nm l ~ 500 nm l ~ 480 nm l ~ 450 nm The different colors of light correspond to different wavelengths and frequencies Continuous Spectra White light passed through a prism produces a spectrum – colors in continuous form. Line Spectra Light passed through a prism from an element produces a discontinuous spectrum of specific colors Line Spectra The pattern of lines emitted by excited atoms of an element is unique = atomic emission spectrum Key Evidence I – Blackbody radiation • Kirchhoff (1859) observed “blackbody” radiation. • What is a black body? What is blackbody radiation? • Spectrum of the intensity (brightness) of the radiation yielded a typical bell curve..SHOCKER Blackbody Radiation Curves Actual Predicted? Planck’s Interpretation of – Blackbody Radiation Studies • Planck (1900) proposed that the vibrating atoms in a heated solid could absorb or emit electromagnetic energy only in discrete amounts; hypothesized that energy is not continuous but existed in discrete bundles called quanta •The smallest amount of energy, a quantum, is given by: E = hv, where h is Planck’s constant: = 6.626 × 10–34 J s •Planck’s quantum hypothesis states that energy can be absorbed or emitted only as a quantum or as whole multiples of a quantum Key Evidence II – Photoelectric Effect •Photoelectric Effect (discovered by Heinrich Hertz; 1887) = the release of electrons from a metal surface when struck by light of “appropriate” frequency •According to classical theory, the intensity of the light shone on the metal impacts the KE of the liberated electrons; the photoelectric effect disprove this however •So what impacted the KE of the liberated electrons? Einstein’s explanation of the Photoelectric Effect • Einstein hypothesized that light was bundled into little packets called photons • The energy of a photon can be likened to the monetary value ascribed to coins • A photon of red light contained less energy than a photon of UV light • Electrons cannot break free unless they absorb a certain minimum quantity of energy from a single photon Bohr’s Hydrogen Atom Niels Bohr found that the electron energy (En) was quantized, that is, that it can have only certain specified values Each specified energy value is called an energy level of the atom The Bohr Model En = –B/n2 where B is a constant = 2.179 × 10–18 J and n is an integer The negative sign represents the forces of attraction The energy is zero when the electron is located infinitely far from the nucleus Energy Levels and Spectral Lines for Hydrogen Bohr Explains Line Spectra Bohr’s equation is most useful in determining the energy change (Elevel) that accompanies the leap of an electron from one energy level to another For the final and initial levels: B Ef 2 nf and B Ei 2 ni The energy difference between nf and ni is: B B 1 1 E 2 2 B 2 2 nf ni ni nf Ground States and Excited States Electrons in their lowest possible energy levels are in the ground state Electrons promoted to any level n > 1 are in an excited state Electrons are promoted by absorbing energy e.g., electric discharge, heat, lasers (photons) Electrons in an excited state eventually drop back down to the ground state “relaxation” The Quantum (Wave) Mechanics Model • In 1924, a French physicist named Louis de Broglie suggested that, like light, electrons could act as both particles and waves. • De Broglie's hypothesis was soon confirmed in experiments that showed electron beams could be diffracted or bent as they passed through a slit much like light could. • The waves produced by an electron confined in its orbit about the nucleus sets up a standing wave of specific wavelength, energy and frequency (i.e., Bohr's energy levels) much like a guitar string sets up a standing wave when plucked. • De Broglie's vision of Bohr's atom Quantum (Wave) Mechanics Quantum mechanics, or wave mechanics, is the treatment of atomic structure through the wavelike properties of the electron Erwin Schrödinger developed an equation to describe the hydrogen atom A wave function is a solution to the Schrödinger equation and represents an energy state of the atom Wave Mechanics = Probability Wave mechanics provides a probability of where an electron will be in certain regions of an atom This region of space where there’s a high probability of finding an electron is called an orbital Wave mechanics led to the idea of a “cloud of electron density” rather than a discrete location Quantum Numbers and Atomic Orbitals A wave function with a given set of these three quantum numbers is called an atomic orbital In quantum mechanics the atomic orbitals require three integer quantum numbers to completely describe the energy and the shape of the 3-D space occupied by the electron (n, l, and ml) Principal Quantum Number (n) • Is independent of the other two quantum numbers • Can only be a positive integer • indicates the size of an orbital (distance from the nucleus) and its electron energy • n can be 1, 2, 3, 4, … Orbital Angular Momentum Quantum Number (l) (aka Azimuthal quantum number) • Determines the shape of the orbital: s, p, d, f , which corresponds to values l values of: 0, 1, 2, 3 • Possible values of l: 0 to n – 1; e.g. if n = 2, l can only be 0 or 1 • Each of these orbitals is in a different region of space and has a different shape •All the ‘l’ quantum values represent different sublevels or subshells •When n = 1, there is only one “l” value meaning there is only one sublevel in the first energy level; when n= 2; there are two values for ‘l’ indicating two sublevels in the second energy level Magnetic Quantum Number (ml) Determines the orientation in space of the orbital; so named because in a magnetic field, these different orientations have different energies Possible values: –l to +l; e.g., if l = 2, ml can be –2, –1, 0, 1, 2 The magnetic quantum number, ml, defines the number of orbitals in a sublevel. E.g. in the l = 0 sublevel, there is only one ml value, therefore there is only orbital in this sublevel; when l=1; there are 3 possible ml values (-1, 0, +1) 3 orbitals in this sublevel Quantum Numbers Summary Taken together the three quantum numbers specific the orbital the electron occupies. Namely: the energy of the orbital, the shape of the orbital, and the orientation of the orbital . • writing 3 quantum numbers to indicate every possible orbital an electron can occupy is cumbersome; instead do we do the following: – retain the numeric value of the principal quantum number and use a letter to indicate the azimuthal quantum number: l = 0 s; l = 1 p; l = 2 d; l = 3 d - When combined, they indicate an a specific orbital e.g. 1s orbital; 2s orbital; 2p orbital Radial Distributions Electrons are most likely to reside nearest the nucleus because of electrostatic attraction Probability of finding an electron decreases as distance (radius) from the nucleus increases Electron Probabilities and the 1s Orbital The 1s orbital looks very much like a fuzzy ball, that is, the orbital has spherical symmetry (the probability of finding an electron is the same in direction) The electrons are more concentrated near the center Electron Probabilities and the 2s Orbital The 2s orbital has two regions of high electron probability, both being spherical The region near the nucleus is separated from the outer region by a spherical node - a spherical shell in which the electron probability is zero EOS The Three p Orbitals -There are three p orbital; each orbital is cylindrically symmetrical with respect to rotation around one of the 3 axes, x, y, or z Each ‘p’ orbital has two lobes of high probability density separated by a node (region of zero probability) The Five d Orbitals Electron Spin (ms) The electron spin quantum number explains some of the finer features of atomic emission spectra The spin refers to a magnetic field induced by the moving electric charge of the electron as it spins Only possible values = –1/2 to +1/2 EOS