B atoms - Electron poor materials
advertisement
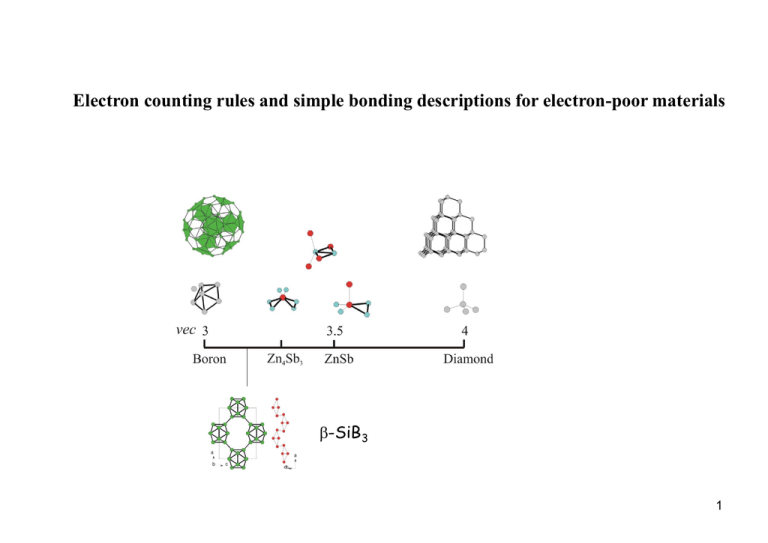
Electron counting rules and simple bonding descriptions for electron-poor materials b-SiB3 1 Boron – the master of clusters a-rhombohedral boron Bn Clusters in halides and hydrides (boranes) B4Cl4 B8Cl8 B9Br9 Icosahedral clusters in elemental B b-rhombohedral boron 2 Boranes Bonding in boranes Hoffmann, R.; Lipscomb, W. N. J. Chem. Phys. 1962, 37, 2872. Wade K. J. Chem. Soc. Chem. Comm. 1971, 792. Wade, K. Inorg. Nucl. Chem. Lett. 1972, 8, 559. 3 B6H62- Point group: Oh Number of electrons: 26 Number of basis functions: 30 1 px 5 2 Local coordinate system 5 4 3 y 2 4 py 6 x 3 Dividing the orbitals: B atoms: H ligand atoms: two type functions (px and py) two type orbitals (s, pz or better: two sp hybrid orbitals, one inward and one outward pointing) one type orbital (s) Constructing MOs: B atoms: H The two sets of skeleton bonding combinations (12 basis functions) transform as: ligand bonding T1g, T2g, T1u, T2u B H H Those combinations correspond already to (triply degenerated) MOs. skeleton bonding The two sets of combinations transform as: A1g, Eg, T1u A1g, Eg, T1u of which one is skeleton bonding (the set of inward pointing sp hybrid orbitals) and thus already represent MOs. H atoms: One set of type SALCs A1g, Eg, T1u Use of 12 basis functions and 12 electrons for terminal ligand bonding, six bonding MOs 4 (a1g, eg, t2u). For skeleton bonding 18 basis functions and 14 electrons remain. B- MO diagram B- MO diagram T1g T2u T1u T2g - and type skeleton MOs with the same symmetry (T1u) interact which leads to a net stabilisation of the borane skeleton. 5 Wade’s rules Wade’s rules link cluster geometries to certain electron counts A closo deltahedral cluster cage (parent poyhedron) with n vertices requires (n+1) pairs of electrons for skeleton bonding. From a parent closo page with n vertices, a set of more open cages (nido, arachno, hypho) can be derived with a formally unchanged skeleton bonding picture Thus, for a parent closo deltahedron with n vertices, the related nido-cluster has (n-1) vertices, but still (n+1) skeleton bonding MOs. Thus, for a parent closo deltahedron with n vertices, the related arachno-cluster has (n-2) vertices, but still (n+1) skeleton bonding MOs. Thus, for a parent closo deltahedron with n vertices, the related hypho-cluster has (n-3) vertices, but still (n+1) skeleton bonding MOs. A entity BH in boranes may be replaced by a entity CH (carboranes) or P. Alternatively: Closo deltahedral clusters with n entities (vertices) (BH, CH, P) are stable with (4n+2) electrons. Nido clusters with n entities are stable with (4n+4) electrons. Arachno clusters with n entities are stable with (4n+6) electrons. 6 Electron counting for aB12 a-B12 36 electrons per icosahedron 26 for skeleton bonding 6 for 2c2e terminal bonding 6x2/3 = 4 for 3c2e bonding within layers 7 a-B12 G. Will et al. (2001) 8 g-B28 Electron counting for g-B28 structure unit linkage B12 4 × 2c2e 8 × 3c2e 26 4 × 2/2 8 × 2/3 B2 2 × 2c2e 4 × 3c2e 2 2 × 2/2 4 × 2/3 9 10 From III-V to II-V semiconductors II III IV V GaSb and ZnSb EN: Sb = 1.7, Ga = 1.7, Zn = 1.6 GaSb ZnSb DEN 0 0.1 Eg [eV] 0.81 direct 0.50 indirect vec 4 3.5 Sb 11 Electronic structure of ZnSb ZnSb – An electron poor framework semiconductor The ZnSb framework has a modest polarity The optimum electron count is 3.5 e/atom Non-classical 4c4e bonding within rhomboid rings Zn2Sb2 (localized multicentre bonding) A. Mikhaylushkin, J. Nylén, U. Häussermann, Chem. Eur. J, 11 (2005), 4912 12 Electronic structure of b-Zn4Sb3 (Zn6Sb5 ) R-3c 36 Zn 18 Sb1 12 Sb2 = Zn36Sb30 (Zn6Sb5 = Zn3.6Sb3) H. W. Mayer, I. Mikhail, K. Schubert, J. Less-Common Met. 59 (1978), 43. Less electrons than ZnSb: rhomboid rings condense into chains electron count Zn6Sb5 = 3 [Zn2Sb12/2] 3 (4 + 4 + 4/2) = 30 2 [Sb2] 2 (4 x 2) = 8 38 e for electron precise conditions (3.454 e/atom); 37 e available 13 b-SiB3 b c a Si42+ B122- 14 b-boron? 15










