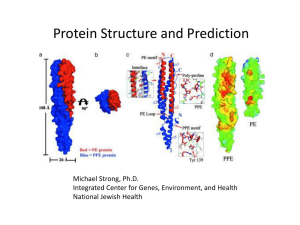
Protein Structures - the University of California, Davis
advertisement

Protein Structures: Experiments and Modeling Patrice Koehl Structural Bioinformatics: Proteins Proteins: Sources of Structure Information Proteins: Homology Modeling Proteins: Ab initio prediction Proteins: Quality of Structures/Models Structural Bioinformatics: Proteins Proteins: Sources of Structure Information Proteins: Homology Modeling Proteins: Ab initio prediction Proteins: Quality of Structures/Models Proteins: Finding the Primary Structure Methods for finding the sequence of a protein: -Translating gene sequence - For proteins from prokaryotes, direct translation - For proteins from eukaryotes, we need the sequence of mRNA or cDNA -Edman degradation limited to “small” proteins, up to 50 amino acids for automated sequencer -Mass spectrometry Proteins: Finding the Primary Structure (Phenyl isothiocyanate or PITC) (Trifluoroacetic acid, TFA) (http://en.wikibooks.org/wiki/Structural_Biochemistry/Proteins/Protein_sequence_determination_techniques) Proteins: Finding the Tertiary Structure Methods for finding the 3D structure of a protein: - Circular Dichroism (low resolution; provides information on secondary structure) -X-ray crystallography (high resolution; finds structure of a protein in a crystal) - NMR spectroscopy (high resolution; finds structure of a protein in solution) Proteins: Circular Dichroism Circular dichroism (CD) spectroscopy measures differences in the absorption of left-handed polarized light versus right-handed polarized light which arise due to structural asymmetry. Different secondary structures in proteins have different CD spectra as they have different asymmetry. CD therefore can detect secondary structures in protein Proteins: X-ray Crystallography Bragg’s Law: 2dsin(q ) = nl Intensity From the “pattern of diffraction”, i.e. the maximum of intensities observed, we can find the angles of diffraction and for each angle we get the corresponding d using Bragg’s law Angle General principle of X-ray crystallography applied to proteins: 1) We need a crystal 2) From the diffraction pattern, we get the crystal organization 3) From the diffraction intensities, we get the electron densities 4) Once the electron density map we fit a structure that matches with this density 5) From the atomic model, we can compute a theoretical diffraction map; if it matches with the experimental one, we are done; otherwise refine Getting the Diffraction Pattern Rosalyn Franklin: Diffraction pattern for DNA From Diffraction to Electron Density Map 3D Fourier Transform One hidden problem: diffraction patterns provide intensities; for Fourier transform, need intensity and phase. A significant step in X-ray crystallography is the solve the “phase problem”. Fitting the structure: Influence of the resolution 2.6 Å resolution 1.2 Å resolution Resolution of X-ray structures Resolution (Å) Meaning >4.0 3.0 - 4.0 2.5 - 3.0 2.0 - 2.5 1.5 - 2.0 0.5 - 1.5 Individual coordinates meaningless Fold possibly correct, but errors are very likely. Fold likely correct except that some surface loops might be mismodelled. Many small errors can normally be detected. Fold normally correct and number of errors in surface loops is small. Water molecules and small ligands become visible. Many small errors can normally be detected. Folds are extremely rarely incorrect, even in surface loops. In general, structures have almost no errors at this resolution. geometry studies are made from these structures. (http://en.wikipedia.org/wiki/Resolution_(electron_density) Large molecular assemblies: X-ray crystallography and Cryo-EM X-ray structure (180 copies of the same protein) (Norwalk virus: http://www.bcm.edu/molvir/norovirus) Large molecular assemblies: X-ray crystallography and Cryo-EM Cryo-EM: -Microscopy technique; as such, do not need crystal (closer to physiological conditions) -Not high-resolution enough to provide atomic details; used in combination with modeling Structural Bioinformatics: Proteins Proteins: Sources of Structure Information Proteins: Homology Modeling Proteins: Ab initio prediction Proteins: Quality of Structures/Models Why we need Homology Modeling? Aim to solve the structure of all proteins: this is too much work experimentally! Solve enough structures so that the remaining structures can be inferred from those experimental structures The number of experimental structures needed depend on our abilities to generate a model. Sequence Space Structure Space Why we need Homology Modeling? Proteins with known structures Unknown proteins Why does Homology Modeling Work? High sequence identity High structure similarity Russell et al. (1997) J Mol Biol 269: 423-439 Homology Modeling: How it works Homology Modeling: How it works Find template Align target sequence with template Generate model: - add loops - add sidechains Refine model Homology Modeling: Input The query, also called target sequence The template structure and sequence (need high quality structure) The sequence alignment between query and template sequence (Probably the most important input!) Homology Modeling: Which program to use? Wallner B, Elofsson A. All are not equal: a benchmark of different homology modeling programs. Protein Sci. 2005 14(5):1315-27. Homology Modeling: Which program to use? 1) Web service: SwissModel http://swissmodel.expasy.org/ 3 modes: - fully automatic - “Alignment mode”: you provide your own target-template alignment - “Project mode”: provides an environment to edit alignment 2) Software: Modeller http://www.salilab.org/modeller/ Probably the best maintained software the homology modeling Structural Bioinformatics: Proteins Proteins: Databases of Models Do not start a homology modeling project before checking… – Swiss-Model repository (http://swissmodel.expasy.org/repository/) • Companion to the Swiss-Model tools – over 3.0 million models of protein domains computed from over 2.2 million sequences. – ModBase (http://modbase.compbio.ucsf.edu/) • Companion to Modeller Structural Bioinformatics: Proteins Proteins: Sources of Structure Information Proteins: Homology Modeling Proteins: Ab initio prediction Proteins: Quality of Structures/Models Secondary Structure Prediction Given a protein sequence a1a2…aN, secondary structure prediction aims at defining the state of each amino acid ai as being either H (helix), E (extended=strand), or O (other) (Some methods have 4 states: H, E, T for turns, and O for other). The quality of secondary structure prediction is measured with a “3-state accuracy” score, or Q3. Q3 is the percent of residues that match “reality” (X-ray structure). Secondary Structure Assignment Determine Secondary Structure positions in known protein structures using DSSP or STRIDE: 1. Kabsch and Sander. Dictionary of Secondary Structure in Proteins: pattern recognition of hydrogen-bonded and geometrical features. Biopolymer 22: 2571-2637 (1983) (DSSP) 2. Frischman and Argos. Knowledge-based secondary structure assignments. Proteins, 23:566-571 (1995) (STRIDE) Early methods for Secondary Structure Prediction Chou and Fasman (Chou and Fasman. Prediction of protein conformation. Biochemistry, 13: 211-245, 1974) GOR (Garnier, Osguthorpe and Robson. Analysis of the accuracy and implications of simple methods for predicting the secondary structure of globular proteins. J. Mol. Biol., 120:97120, 1978) Chou and Fasman Start by computing amino acids propensities to belong to a given type of secondary structure: P(i / Helix ) P (i ) P(i / Beta ) P (i ) P(i / Turn) P (i ) Propensities > 1 mean that the residue type I is likely to be found in the Corresponding secondary structure type. Chou and Fasman Amino Acid Ala Cys Leu Met Glu Gln His Lys Val Ile Phe Tyr Trp Thr Gly Ser Asp Asn Pro Arg -Helix 1.29 1.11 1.30 1.47 1.44 1.27 1.22 1.23 0.91 0.97 1.07 0.72 0.99 0.82 0.56 0.82 1.04 0.90 0.52 0.96 -Sheet 0.90 0.74 1.02 0.97 0.75 0.80 1.08 0.77 1.49 1.45 1.32 1.25 1.14 1.21 0.92 0.95 0.72 0.76 0.64 0.99 Turn 0.78 0.80 0.59 0.39 1.00 0.97 0.69 0.96 0.47 0.51 0.58 1.05 0.75 1.03 1.64 1.33 1.41 1.23 1.91 0.88 Favors -Helix Favors -strand Favors turn Chou and Fasman Predicting helices: - find nucleation site: 4 out of 6 contiguous residues with P()>1 - extension: extend helix in both directions until a set of 4 contiguous residues has an average P() < 1 (breaker) - if average P() over whole region is >1, it is predicted to be helical Predicting strands: - find nucleation site: 3 out of 5 contiguous residues with P()>1 - extension: extend strand in both directions until a set of 4 contiguous residues has an average P() < 1 (breaker) - if average P() over whole region is >1, it is predicted to be a strand Chou and Fasman f(i) Position-specific parameters for turn: Each position has distinct amino acid preferences. Examples: -At position 2, Pro is highly preferred; Trp is disfavored -At position 3, Asp, Asn and Gly are preferred -At position 4, Trp, Gly and Cys preferred f(i+1) f(i+2) f(i+3) Chou and Fasman Predicting turns: - for each tetrapeptide starting at residue i, compute: - PTurn (average propensity over all 4 residues) - F = f(i)*f(i+1)*f(i+2)*f(i+3) - if PTurn > P and PTurn > P and PTurn > 1 and F>0.000075 tetrapeptide is considered a turn. Chou and Fasman prediction: http://fasta.bioch.virginia.edu/fasta_www/chofas.htm The GOR method Position-dependent propensities for helix, sheet or turn is calculated for each amino acid. For each position j in the sequence, eight residues on either side are considered. j A helix propensity table contains information about propensity for residues at 17 positions when the conformation of residue j is helical. The helix propensity tables have 20 x 17 entries. Build similar tables for strands and turns. GOR simplification: The predicted state of AAj is calculated as the sum of the positiondependent propensities of all residues around AAj. GOR can be used at : http://abs.cit.nih.gov/gor/ (current version is GOR IV) Accuracy Both Chou and Fasman and GOR have been assessed and their accuracy is estimated to be Q3=60-65%. Secondary Structure Prediction -Available servers: - JPRED : http://www.compbio.dundee.ac.uk/~www-jpred/ - PHD: http://cubic.bioc.columbia.edu/predictprotein/ - PSIPRED: http://bioinf.cs.ucl.ac.uk/psipred/ - NNPREDICT: http://www.cmpharm.ucsf.edu/~nomi/nnpredict.html - Chou and Fassman: http://fasta.bioch.virginia.edu/fasta_www/chofas.htm -Interesting paper: - Rost and Eyrich. EVA: Large-scale analysis of secondary structure prediction. Proteins 5:192-199 (2001) Protein Structure Prediction One popular model for protein folding assumes a sequence of events: ◦ Hydrophobic collapse ◦ Local interactions stabilize secondary structures ◦ Secondary structures interact to form motifs ◦ Motifs aggregate to form tertiary structure Protein Structure Prediction A physics-based approach: - find conformation of protein corresponding to a thermodynamics minimum (free energy minimum) - cannot minimize internal energy alone! Needs to include solvent - simulate folding…a very long process! Folding time are in the ms to second time range Folding simulations at best run 1 ns in one day… The Folding @ Home initiative (Vijay Pande, Stanford University) http://folding.stanford.edu/ The Folding @ Home initiative Folding @ Home: Results 100000 Experiments: villin 10000 Predicted folding time (nanoseconds) villin: Raleigh, et al, SUNY, Stony Brook BBAW beta hairpin 1000 100 BBAW: Gruebele, et al, UIUC beta hairpin: Eaton, et al, NIH alpha helix 10 alpha helix: Eaton, et al, NIH PPA 1 1 10 100 1000 10000 experimental measurement (nanoseconds) 100000 PPA: Gruebele, et al, UIUC http://pande.stanford.edu/ Protein Structure Prediction DECOYS: Generate a large number of possible shapes DISCRIMINATION: Select the correct, native-like fold Need good decoy structures Need a good energy function Structural Bioinformatics: Proteins Proteins: Sources of Structure Information Proteins: Homology Modeling Proteins: Ab initio prediction Proteins: Quality of Structures/Models What is a “good” protein structure? -The structure must have good stereochemistry -Bond lengths and bond angles close to ideal values -Backbone dihedral angles in “allowed” regions of the Ramachandran plot -Side-chains close to “rotamer” states -The structure should be energetically favorable - No “clashes” (such as two atoms too close to each other) - Hydrophobic residues should be mostly buried - Polar residues should be on the surface. No charges buried. I advise running Procheck to check the quality of a protein structure: -As a standalone program: http://www.ebi.ac.uk/thornton-srv/software/PROCHECK/ -Or through the PDBsum web portal: http://www.ebi.ac.uk/pdbsum/ What have we learnt? X-ray crystallography is the method of choice for the elucidation of the high-resolution structure of a protein. Alternative methods include NMR and Cryo-EM. The mapping between protein sequence and protein structure is not one-to-one: for one sequence, there is (usually) one structure, but for one structure, there can be many sequences What have we learnt? Homology modeling is a computational technique that predicts the structure of a target protein based on a template structure and an alignment between the target and template sequences. The quality of the alignment defines the quality of the homology models. Two good programs for homology modeling: Swiss-Model (web-based) and Modeller (standalone software) The swiss model repository and ModBase are useful databases of protein structure models generated by homology modeling. It is usually good practice to check the quality of a protein structure or model. ProCheck is a useful tool for this: it checks both the stereochemistry and energetics of the protein.