Isoelectric Focusing
advertisement
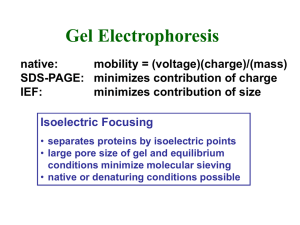
Isoelectric Focusing • Technique combining ideas of isoelectric points and electric fields • Very high resolution technique for protein 1,3 pI • Isoelectric focusing uses the theory of protein pI – pI is the pH at which a given protein has a neutral overall charge • The pI is dependant on which type of residues are present and how many – Bases make proteins positive and acids negative • pI is very specific for each protein 1 How to Isoelectrofocus • • • • Establish a pH gradient Establish a voltage Stain your macromolecule (usually protein) Go do something while proteins move 1 What Happens • Protein is loaded at the top of a column where pH is very high – Most things are negatively charged at this pH – Protons are stripped from residue side chains • Proteins move in the electric field toward the distant cathode and away from the nearby anode • As the proteins move through the pH gradient, they gain positive charge and reach neutrality • At pH=pI, the proteins have no charge and stop 1 What Happens • Proteins stop exactly at pH=pI and the stained proteins are very visible 1,4 Making a Gradient • Column filled with lowdensity gel to allow proteins to move • Highly stable ampholytes are molecules with specific pKa to give a specific and unchanging pH gradient 1,2 Why Use IEF • We don’t use it for protein purification, affinity chromatography has taken over that area • Can be used 2D with PAGE to see if a certain protein is present in a sample • May be evidence that cells use it to move proteins around, especially with phosphorylation of macromolecules (gives negative charge) 1 References 1. Voet, D. Voet, J. G. Pratt. C. W. Fundamentals of Biochemistry: Life at the Molecular Level. 3rd edition. John Wiley and Sons. (2008) Pictures: 2. http://www.science-tube.com/ 3. http://www.zeitnews.org/ 4.http://www.biochem.arizona.edu/classes/bioc462/4 62a/NOTES/Protein_Properties/protein_purificati on.htm