3 chapter 3_S10_part1 STUDENT
advertisement

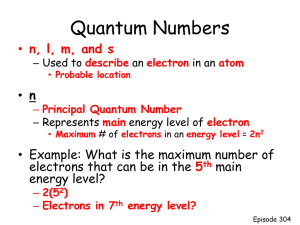
Quantum Theory and the Electronic Structure of Atoms 1 Once scientists developed a logical order for the elements they began studying the structure and composition of individual atoms. They used substances’ interactions with light to explain the structure of atoms and develop a model to explain how atoms affected properties of light. In order to understand interactions, we must understand behavior of light. Light is typically described as traveling in waves (similar to water); All electromagnetic (EM) waves (including light) are made of two components: electric and magnetic EM waves travel at the speed of light, c (2.997924 x 108 m/s ≈ 3.00 x 108 m/s) c = ln (Know these variables!) c = speed of light; l (lambda) = wavelength (m, nm); n (nu) = frequency (1/s, s-1, Hz) EM waves Different colors of light correspond to different wavelengths in the visible portion of the EM spectrum. Two wavelengths (l) are shown below. Determine the frequency (n) for each wave. Blue light Red light 1 nm = 1 x 10-9 m OR 1 x 109 nm = 1 m Classical descriptions: ◦ Dalton: atoms are hard particles, all atoms of the same element are the same ◦ Thomson: atoms are divisible (electrons in atoms) ◦ Rutherford: positively charged nucleus New view of atomic behavior ◦ Planck: Blackbody radiation – heat solids to red or white heat, matter did not emit energy continuously; in whole-number multiples of certain quantities ◦ Matter absorbs or emits energies in packets quanta Quantum has come to mean small; originated from Planck’s observation of quantized energy Einstein used this theory to observe metals reacting to different colors of light – Photoelectric Effect: electrons are ejected from the surface of certain metals exposed to light at a certain minimum frequency ◦ Blue light (n = 6.7 x 1014 Hz) causes Na to emit electrons, red light (n = 4.0 x 1014 Hz) does not Photoelectric Effect Based on photoelectric effect, light acts as a wave but also exists as a stream of particles called photons Energy of photons is proportional to frequency, inversely proportional to wavelength hc E hn -34 J•s h = 6.626 x 10 l J = kg • m2 / s2 1) Which has a higher frequency: light from a red stoplight with a wavelength of 750 nm or a yellow light with a wavelength of 600 nm? 2) What is the wavelength of a radio station’s waves transmitting at a frequency of 101.5 MHz (megahertz)? (FM radio waves range from 30 – 300 MHz.) 3) Red lights at traffic stops have wavelengths of about 650 nm. What is the frequency (in Hz) of this light? 4) Calculate the energy (in Joules) of a photon with a wavelength of 5.00 x 104 nm (infrared region). Answers: yellow, 2.956 m, 4.62 x 1014 Hz, 3.98 x 10-21 J de Broglie: If light can behave like a wave and a particle, then matter (i.e., electrons) can behave like a wave If an electron behaves like a standing wave, then it can only have specific wavelengths Can calculate wavelength for matter if we know its velocity (use v instead of c): l = h / m v (This is the de Broglie equation.) ◦ h = Planck’s constant, m = mass (electron’s have constant mass: 9.11x10-31 kg), v = velocity (speed) The energy of a photon is 5.87 x 10-20 J. What is the frequency of the photon? What is the wavelength of an electron that travels at 34.7 m/s and has a mass of 9.11 x 10-31 kg? A 0.143 kg baseball is thrown at a velocity of 42.5 m/s. Calculate the wavelength of the baseball. How does the baseball’s wavelength compare to the electron from the example above? Calculate the energy of a photon that has a wavelength of 35.6 nm (in the x-ray region). (Hint: Watch units!!!) If electrons have wavelike properties, then we can’t know both its position and velocity. In order to determine the position of an electron, we hit it with a photon of light, but this will change its position and velocity. Bohr sought to reconcile these views of the electron. ◦ Developed the planetary analogy of atoms. ◦ Electrons orbit around the nucleus like planets around the sun. ◦ Electrons travel in discrete, quantized circular orbits; like going up or down stairs. ◦ Each orbit has a specific energy associated with it, labeled as n = 1, 2, etc. ◦ Ground state is the lowest energy level for an atom (n = 1). When an atom absorbs energy, an electron can jump from a lower energy level to a higher energy level. When an atom emits (releases) energy, an electron drops from a higher energy level to a lower energy level. This process sometimes gives off energy as visible light. H e- transitions Eng. Color White light we see consists of all colors in the visible spectrum. Use a prism (or CD) to break them up. cont. spect. white light Light given off by atoms doesn’t necessarily correspond to all visible colors. Flame tests Hydrogen Each element gives off unique spectrum Demo: Gas Discharge Tubes ◦ Each element has its own individual emission spectrum. This allowed scientists to identify elements in different minerals. Spectra of Elements: http://www.wwnorton.com/college/chemistry/chemconnections/BlueLight/pages/elements.html Figure 7.8 The Bohr model worked well for hydrogen, but failed for elements with more than one proton and one electron. Quantum Mechanics was developed (by Schrödinger in the 1920’s) to describe the motion of subatomic particles ◦ Did not attempt to describe position of particles; used mathematical equations to describe the probability of finding the particles ◦ The probability density (map of likely locations) is the “electron cloud” Quantum Mechanics Movie The region of highest probability for finding an electron is an “electron cloud”. This region of high probability is called an atomic orbital. Each orbital holds at most 2 electrons. Modern atom.exe e- orbit vs e- cloud 22 There are 4 quantum number that describe the size, shape, and location of electrons We use these numbers to describe where electrons are found for an atom. Can also use the periodic table!!! The Principal Quantum Number, n ◦ describes distance of the electron from the nucleus; called shells ◦ n = 1, 2, 3, etc; larger number is farther from nucleus ◦ n corresponds to a row in the periodic table 23 The Angular Momentum Quantum Number, l The Magnetic Quantum Number, ml ◦ describes the orientation of the orbital with respect to x, y, ◦ In each row of the periodic table are different groups of orbitals with different shapes. These groups of orbitals are called subshells and labeled s, p, d, and f. ◦ s subshells are spherical (first two columns) ◦ p subshells are dumb-bell shaped (last six columns) ◦ d subshells are intersecting dumb-bells (transition metals) and z axes ◦ s, p, and d orbitals have different shapes and therefore different orientations The Spin Quantum Number, ms ◦ describes the spin of an electron in an orbital (shown as up and down arrows in orbital diagrams) 24 s orbitals are spherical; white rings are nodes (regions where an electron won’t be found) ◦ 1 s orbital in a subshell s orbital 25 2px orbital 2py orbital 2pz orbital p orbitals are dumb-bells (2 lobes); node between lobes ◦ 3 p orbitals in a subshell p orbital 26 d orbitals: intersecting dumb-bells (4 lobes); nodes between lobes ◦ 5 d orbitals in a subshell d orbital 27 The first shell (row) has 1 subshell (s) ◦ s only 1 orbital ◦ An s subshell can hold at most 2 electrons The 2nd shell (row) has 2 subshells (s and p) The 3rd shell (row) has 3 subshells (s, p, and d) The 4th shell (row) has 4 subshells (s, p, d, and f) ◦ p set of 3 orbitals ◦ A p subshell can hold at most 6 electrons ◦ d set of 5 orbitals ◦ A d subshell can hold at most ? electrons ◦ f set of 7 orbitals ◦ What is the maximum number of electrons allowed in the f subshell? 28 Arrangement of subshells in the Periodic Table 29 What is the maximum number of: ◦ ◦ ◦ ◦ ◦ ◦ electrons allowed in the 2px orbital? subshells allowed in the 4th shell? electrons allowed in the 3d subshell? electrons allowed in the 4d subshell? electrons allowed in the 3p subshell? electrons allowed in the 3rd shell? In hydrogen, all shells are equivalent in energy. In many-electron models, the energy levels depend on the shell and subshell. Aufbau principle: start with the nucleus and empty orbitals, then “build” up the electron configuration using orbitals of increasing energy. 33 Arrangement of subshells in the Periodic Table 34 Arrangement of subshells in the Periodic Table 35 Arrangement of subshells in the Periodic Table 36 Arrangement of subshells in the Periodic Table 37 Arrangement of subshells in the Periodic Table 38 Write electron configurations for the following atoms. H He Li Be B N O Ne Na Al S Ar K Sc Ti Zn Br Electrons in the outermost shell. ◦ 1s2 2s2 2p6 ◦ 1s2 2s2 2p6 3s2 3p5 Identify the valence electrons (v. e.) in the following configurations: ◦ 1s2 2s2 2p6 3s2 ◦ 1s2 2s2 2p33s2 1s2 2s2 41 Rather than writing out complete electron configurations, we can use the previously filled shell (noble gas) and show the valence electrons (v. e.): P: 1s2 2s2 2p6 3s2 3p3 [Ne] 3s2 3p3 (5 v. e.) Write the shorthand notation for: ◦ ◦ ◦ ◦ Ca Cl Sr Fe Some exceptions to the Aufbau order… What are the expected electron configurations for Cr and Cu? Filled and half-filled d subshells seem to be especially stable. Cr: 1s2 2s2 2p6 3s2 3p6 4s1 3d5 ◦ Also true for Mo and W Cu: 1s2 2s2 2p6 3s2 3p6 4s1 3d10 ◦ Also true for Ag and Au e_config. 43 If two or more orbitals (i.e., a p or d orbital) with the same energy are available, one electron goes into each orbital until they have to pair up. ◦ Fighting sibling analogy For example, an atom with 2 p electrons: 1 electron will go into the first (px) orbital, the next electron will go into the second (py) orbital. Pauli Exclusion Principle: no two electrons can have the same values of all 4 quantum numbers Describes what happens when electrons share an orbital. ◦ Only two electrons can occupy a single orbital and they must have opposite spin (i.e., the 4th quantum number). The first electron is designated as positive spin (up arrow), the second electron in that orbital has negative spin (down arrow). 45 Orbital diagrams are pictorial representations of electron configurations. Electron Configurations 46 Write electron configurations for the following elements (long-hand notation). Indicate the number of v.e. for each element. potassium sulfur carbon magnesium lithium