File
advertisement

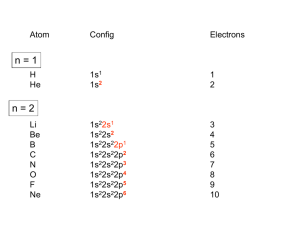
Sub-Topics 1 2 3 4 5 6 • Introduction to Transition Metals • Crystal Field Theory, Spectrochemical Series, Ligand Field Stabilisation Energies • Classical Complexes • Molecular Orbital Theory of Bonding • Jahn – Teller Distortions, Square Planar Complexes • Magnetism: Spin only formula and Electronic Spectroscopy Lecture 7 Molecular Orbital Theory Molecular Orbital Theory Orbital Overlap A bond can only be formed when two atomic orbitals of two atoms overlap Orbitals must be of similar energy To simplify the theory in our context, the 4s and 4p orbitals on the metal atom are ignored. Recall the different types of donor/acceptor behaviour Sigma Donor Ligands point along axis Example, Cl- Sigma Donor For the Cl- ion: Electrons are in a p-orbital Can be donated to form a sigma bond by overlapping with eg orbitals Metal based eg raised in energy No net overlap with t2g Hence energy of t2g unchanged Sigma Donor Molecular Orbital Diagram Effect of Sigma Donation What happens as strength of sigma donation increases? Energy of interaction inter-action Splitting between the bonding and anti-bonding orbitals gets larger eg moves higher in energy Ligand field splitting (Δo) increases Pi donor Certain p-orbitals can interact with t2g to give pi bonds Overlap is possible Example Cl- No net overlap with eg sets Pi donor Molecular Orbital Diagram Effect of Pi Donation What happens when Pi Donor interactions increases? Energy of inter-actions increases Splitting between bonding and anti-bonding orbitals increases t2g moves higher in energy Ligand field splitting (Δo) decreases Complete Molecular Orbital Diagram Combination of sigma and pi donor interactions Pi acceptor Metal orbitals involved are the same as pi donor Symmetry of atomic orbital overlap determines sigma or pi interaction Only interacts with t2g Pi acceptor Requirements Ligand must have an empty or partially filled orbital of pi symmetry Accepting electron density Typically anti-bonding orbitals Results in Back Donation Examples, CO and CN- Pi acceptor Molecular Orbital Diagram Pi acceptors increases Δo Lecture 8 Effects of Ligand Field Splitting The following properties of transition metal complexes are effected under Ligan d Field Splitting (ie introduction of ligands) Thermochemical Data Hydration Energies of the M(H2O)62+ ions Redox potentials for M3+/M2+ Lattice Enthalpies Ionic Radii Coordination Geometries Effects of Ligand Field Splitting Hydration Energy What factors effect the hydration energy? Zeff, Ionic Radius, Ligand Field Effects? In each case we are going to deal with the hexaaquo ion M(H2O)62+ Ligand Field Symmetry: Octahedral Calculate Ligand Field Stabilisation Energies – reflects the double hump! Electrode Potentials for M3+/M2+ Ligands alter the redox potential Example Fe3+/Fe2+couple Ligand (o-phentanthroline)3 (H2O)6 E (V) +1.10 +0.71 Ligand field effects alter the redox potential Differences in Δo for water and other ligand Changes in spin state and hence LFSE Our Favourite Friend Again… Energy Cycle Determining change in energy through introduction of a ligand Electrode Potentials Ligand changes alter redox potential Stabilises one oxidation state over another! LFSE is a small component in Δgcomplex Not to do with spin state as remains same Ligand Spin State (o-phenanthroline)3 Both Low Spin (H2O)6 Both High Spin Electrode Potentials LFSE plays a part Ligand Δo / cm-1 o-phen 20000 H2O 11000 Complex more stable with o-phen through LFSE Metal-Ligand bond energies favour M2+ o-phen Ionic Radii What do we already know Decrease as you go across row () as Zeff increases Electrons are placed in t2g, non-bonding orbitals Why increase to Mn2+? Filling of eg orbitals are of anti-bonding character Lattice Enthalpies Sub-Topics 1 2 3 4 5 6 • Introduction to Transition Metals • Crystal Field Theory, Spectrochemical Series, Ligand Field Stabilisation Energies • Classical Complexes • Molecular Orbital Theory of Bonding • Jahn – Teller Distortions, Square Planar Complexes • Magnetism: Spin only formula and Electronic Spectroscopy Lecture 8 General Principles High Co-ordination seen at beginning of transition series Metal atoms have larger radii Fewer electrons making it easier to accept electrons in sigma donation SIX CO-ORDINATION Low Co-ordination seen at end of transition series Metal ions are smaller Rich in d-electrons NOT SENSIBLE TO ASSIGN COORDINATION DUE TO LIGAND FIELD EFFECTS Ligand Field Effects Jahn-Teller Distortions Cr and Cu did not give regular octahedral environments in metal oxides MO (M2+) Co-ordination Symmetries Ni, Pd, Pt either triad, square planar, or tetrahedral Jahn-Teller Distortions If ground electronic state of a non-linear complex is orbitally degenerate the complex will distort to remove the degeneracy When do you get Jahn-Teller Distortions Odd number of electrons in the eg or t2g other than 3 in the t2g Notes Nature of distortion is not defined Axial lengthening through to square planar Structural distortions more evident with odd number of eg Antibonding Orbitals Tetragonal Distortion How is orbital degeneracy lifted? Sub-Topics 1 2 3 4 5 6 • Introduction to Transition Metals • Crystal Field Theory, Spectrochemical Series, Ligand Field Stabilisation Energies • Classical Complexes • Molecular Orbital Theory of Bonding • Jahn – Teller Distortions, Square Planar Complexes • Magnetism: Spin only formula and Electronic Spectroscopy Magnetism Magnetic properties of a complex can tell us: • Electronic State of the transition metal • Co-ordination geometry Free atom or ion Consists of orbital angular momentum of the electron L, and the spin angular momentum of the electron S. Complex Orbital angular momentum is quenched! Spin-only formula μ = 2 𝑆(𝑆 + 1) μ = 2 𝑁(𝑁 + 2) Unit: Bohr Magneton B.M or μB Magnetic Moment of a complex can be interpreted to number of unpaired delectrons it has Deviations from Spin-Only Presence of orbital angular momenum Electron circulation not fully quenched High spin/Low spin crossover Two ways this can happen Change in the ground state Low spin at low temperature and high spin at high temperature Electronic States for high and low spin close in energy Therefore thermal population of the two spin states with temperature Lecture 9 Electronic Spectra Selection Rules Spin Lowest Intensity ∆𝑆 = 0 Orbital Momentum ∆𝐿 = ∓1 Laporte 𝑢 ↔ 𝑔 𝐴𝑙𝑙𝑜𝑤𝑒𝑑 𝑔 ↔ 𝑔 𝑎𝑛𝑑 𝑢 ↔ 𝑢 𝐹𝑜𝑟𝑏𝑖𝑑𝑑𝑒𝑛 𝑇ℎ𝑒𝑟𝑒𝑓𝑜𝑟𝑒 𝑡2𝑔 ↔ 𝑒𝑔 𝐹𝑜𝑟𝑏𝑖𝑑𝑑𝑒𝑛 Charge Transfer Highest Intensity Both spin and orbitally allowed