Bioph 702-Patch clamp-Mangel-handout
advertisement
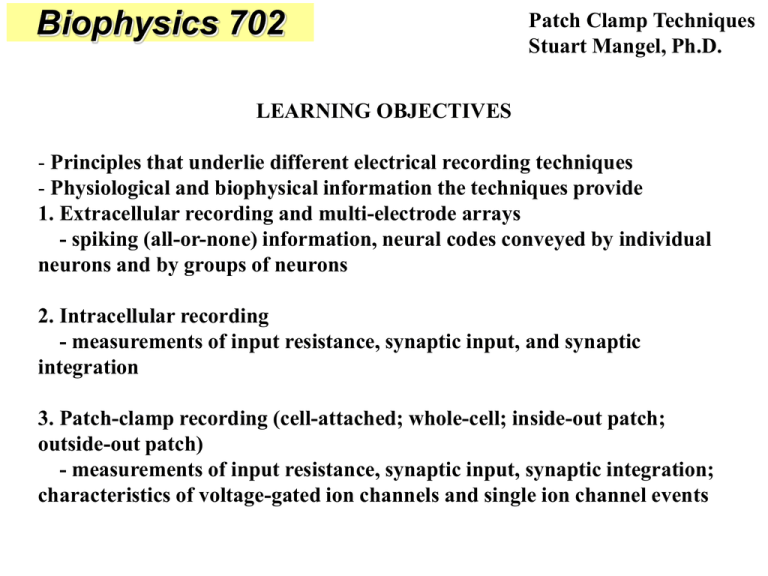
Biophysics 702 Patch Clamp Techniques Stuart Mangel, Ph.D. LEARNING OBJECTIVES - Principles that underlie different electrical recording techniques - Physiological and biophysical information the techniques provide 1. Extracellular recording and multi-electrode arrays - spiking (all-or-none) information, neural codes conveyed by individual neurons and by groups of neurons 2. Intracellular recording - measurements of input resistance, synaptic input, and synaptic integration 3. Patch-clamp recording (cell-attached; whole-cell; inside-out patch; outside-out patch) - measurements of input resistance, synaptic input, synaptic integration; characteristics of voltage-gated ion channels and single ion channel events EXTRACELLULAR VS. INTRACELLULAR RECORDING Extracellularly and intracellularly recorded voltages are in the microvolt and millivolt ranges, respectively. Maintaining the resting membrane potential The Goldman-Hodgkin-Katz (GHK) Equation: The steady state membrane potential for a given set of ionic concentrations inside and outside the cell and the relative permeability of the membrane to each ion RT pK[K+]o + pNa[Na+]o + pCl[Cl-]i Vm = ln F pK[K+]i + pNa[Na+]i + pCl[Cl-]o -60 to -75 mV extracellular ENa = +56 Na+ (150) intracellular Na+ (18) EK = -102 K+ (3) K+ (135) ECl = -76 Cl- (120) ECa = +125 Ca2+ (1.2) Cl- (7) Ca2+ (0.1 µM) NSCC Na+,K+-ATPase INTRACELLULAR RECORDING Measuring EM • Measure the potential difference between two electrodes using a D.C. amplifier • Expected value of the membrane potential is in millivolts (not microvolts), so the gain does not need to be as high Intracellular Recording • When a fine-tipped electrode penetrates the membrane of a cell, one observes a sudden change in the measured potential to a more negative value. • Typical problems – High impedance μE – Damage when cell penetrated MEASURING THE INPUT RESISTANCE Wheatstone Bridge • Used to measure an unknown resistance • Discovered by Hunter Christie, 1833 • Popularized by Charles Wheatstone BALANCING THE BRIDGE • • • • R1 = Fixed R R2 = Variable R R3 = Fixed R R4 = Unknown R To get R2/R1 = R4/R3, adjust R2, so that there is no current across B, C R4 = (R2/R1)·R3 CALCULATING THE INPUT RESISTANCE OF A CELL “Balanced” 0 Membrane Potential (mV) • Balance the bridge before entering the cell • After impaling the cell, the bridge is “out of balance” by the R value of the cell • I is known, measure V, and calculate R using Ohm’s Law (V = IR) • R = V/I “Out of Balance” -20 APPLY DRUG -40 Did R increase or decrease? -60 Did channels open or close? -80 -100 0 100 200 300 Time (Arbitrary Units) 400 500 PATCH-CLAMP RECORDING • Neher and Sakmann, Nobel Prize, 1991 • Tremendous technical breakthrough that improved the signal to noise ratio of the recording • Record from whole cells or from a small patch of cell membrane, so only a few ion channels (or one) can be studied • High resistance (in giga-ohms) and high mechanical strength of the seal between the glass electrode and the cell membrane enable one to observe very small currents. • The diameter of the tip of patch electrodes can be larger than that of fine-tipped intracellular microelectrodes (1.0 micron vs. 0.05 microns), so that the resistance of patch electrodes is lower (e.g. 5 MΩ vs 200 MΩ). The lower resistance of patch electrodes makes voltage clamping easier. Patch clamp recording configurations Electrode Glass pipette Ion channel Plasma membrane Perforated-patch antibiotics Cell-attached pull Inside-out suction Whole-cell pull Outside-out SUMMARY OF ADVANTAGES AND DISADVANTAGES OF PATCH CLAMP CONFIGURATIONS THE VOLTAGE CLAMP THE ACTION POTENTIAL Voltage clamping reveals the ionic currents that underlie the action potentials observed in squid axons Activation and Inactivation Properties Ionic Selectivity SODIUM CHANNEL GATING CURRENT Reversal potentials for synaptic currents Inhibitory actions of GABA synapses result from the opening of ion channels selective for Cl- ROLE OF ION TRANSPORTERS IN NEURAL NETWORK FUNCTION Fig. 1 GABA-evoked depolarization GABA-evoked hyperpolarization Fig. 1. The chloride cotransporters, Na-K-2Cl (NKCC) and K-Cl (KCC2), determine whether the neurotransmitter GABA, which opens Cl- channels, depolarizes or hyperpolarizes neurons, respectively. Fig. 2 Fig. 3 Fig. 3. The GABA reversal potential at the starburst amacrine cell (SAC) distal dendrite is more hyperpolarized than at the proximal dendrite due to KCC2 activity. (A, B) GABA was applied onto the proximal dendrite (A) and onto the distal dendrite (B) ~ 100 m from the cell body of a SAC in the presence of cobalt (2 mM) to block synaptic transmission. (C) Average EGABA of the proximal and distal dendrites of SACs were significantly different (p < 0.01). (D) Average EGABA of distal dendrites before and during bath application of FUR (25 M), a selective inhibitor of KCC2 activity, were significantly different (p < 0.01). Fig. 2. The dendrites of starburst amacrine cells (green), a type of interneuron in the retina, hyperpolarize to light stimuli that move from the periphery to the cell body (bottom left) and depolarize to light stimuli that move from the cell body to the periphery (bottom right). These directionally-selective responses are generated in part by the differential distribution of the Na-K-2Cl (NKCC) cotransporter (pink) on the cell body and proximal dendrites and the K-Cl (KCC2) cotransporter (blue) on the distal dendrites. The expression patterns of Na-K-2Cl and K-Cl are represented as pink to purple and purple to blue gradients, respectively, on the dendrites and cell body of this starburst cell. - modified from Gavrikov et al., 2006, PNAS SODIUM CHANNEL CURRENTS RECORDED FROM CELL-ATTACHED PATCH Properties of AChgated channels Single open ACh-gated channels behave as simple resistors. Extracellular Mg2+ ions block NMDA channels under physiological conditions. Questions: Stuart Mangel, Ph.D. Professor Department of Neuroscience The Ohio State University College of Medicine 614-292-5753 mangel.1@osu.edu Readings: Kandel, Schwartz & Jessell, Principles of Neural Science – Chaps. 9, 11 & 12 Squire, Berg et al., Fundamental Neuroscience – Chaps. 6 & 11