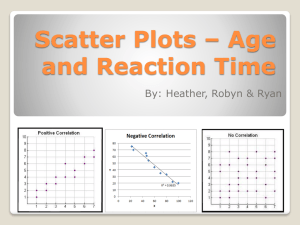
Flow-cytometry
advertisement
Payvand Clinical Specialty Lab. FLOWCYTOMETRY Behzad Poopak, DCLS PhD bpoopak@yahoo.com What Is Flow Cytometry? Flow ~ cells in motion Cyto ~ cell Metry ~ measure Measuring properties of cells while in a fluid stream Cytometry vs. Flow Cytometry Cytometry Localization of antigen is possible Poor enumeration of cell subtypes Limiting number of simultaneous measurements Flow Cytometry. Cannot tell you where antigen is. Can analyze many cells in a short time frame. Can look at numerous parameters at once. Applications of Flow Cytometry. • Cell size. • Cytoplasmic granularity. • Cell surface antigens (Immunophenotyping). • Apoptosis. • Intracellular cytokine production. • Intracellular signalling. • Gene reporter (GFP). • Cell cycle, DNA content, composition, synthesis. • Bound and free calcium. • Cell proliferation • Cell sorting, single cell cloning Flow cytometry & Hematopathology 1. Distinction between neoplastic and benign conditions, 2. Diagnosis and characterization of lymphomas and leukemias, 3. Assessment of other neoplastic and preneoplastic disorders such as plasma cell dyscrasias and MDS, 4. Detection of MRD in patients with acute leukemia or chronic lymphoid malignancies. 5. In some groups of lymphoid neoplasms, FCM study also provides prognostic information. Principle of Flow Cytometry Fluidics • Cells in suspension • Cells flow in single-file • Intercepted by light source(s) (laser) Optics • Scatter light and emit fluorescence • Signal collected, filtered and • Converted to digital values Electronics • Storage on a computer Data display and analysis Basic Principles of Flow Cytometry Single cell or particle suspension Fluorescent dyes or Abs that can be attached to an antigen or protein of interest Flow cell, sheath fluid and a focused laser beam The Flow Cell Sheath Cell Sample Stream The introduction of a large volume into a small volume in such a way that it becomes “focused” along an axis is called Hydrodynamic Focusing. Sample Sample Sheath Sheath Laser Focal Point Sheath Sample Core Stream Incoming Laser Low Differential High Differential Sample Differential 10 psi 10 psi 10 psi 10.2 psi 10.4 psi 10.8 psi Difference in pressure between sample and sheath This will control sample volume flow rate The greater the differential, the wider the sample core. If differential is too large, cells will no longer line up single file Results in wider CV’s and increase in multiple cells passing through the laser at once. No more single cell analysis! Basic Principles cont’d Light is either scattered or absorbed when it strikes a cell Light scatter is dependent on the internal structure, size and shape. Forward scatter = size of the cell Side Scatter = complexity of the cell Forward Scatter Laser Beam FSC Detector Side Scatter Laser Beam FSC Detector Collection Lens SSC Detector Side scatter Granulocytes Monocytes Lymphocytes Forward scatter Why Look at FSC v. SSC Since FSC ~ size and SSC ~ internal structure, a correlated measurement between them can allow for differentiation of cell types in a heterogeneous cell population Granulocytes SSC Lymphocytes Monocytes RBCs, Debris, Dead Cells FSC Low Medium High levels of forward scatter Side scatter ----> increasing cell size Forward scatter Side scatter Medium Low Forward scatter Increasing levels of side scatter ----> increasing cell granularity High BY “GATING” EACH OF THE AREAS IN 2 DIMENSIONS, YOU CAN ADAPT FLOW CYTOMETRY TO PERFORM DIFFERENTIAL COUNTS! Side scatter Granulocytes Monocytes Lymphocytes Forward scatter FLOW CYTOMETRY - Cells are labeled with fluorescent antibodies directed against cell surface molecules - Using different color fluorochromes allows counting of many markers simultaneously and allows identification of several markers on the same cell ( Multiparameter Flow) - In the instrument, cells pass one-by-one past a laser to excite the fluorochromes and there are detectors for each type of fluorochrome - cells are labeled with fluoresence antibodies Flouresent tag Surface of a cell, e.g., a lymphocyte (in solution) Fluorescence Photon emission as an electron returns from an excited state to ground state What Happens in a Flow Cytometer (Simplified) Fluorochrome Basic Principles cont’d Fluorescent dyes absorb light of a specific wavelength and reemit light of a different wavelength Fluorescent signals are detected by PMT and amplified Optical filters are used to steer light of specific wavelengths to the photo detector Reflected Dichroic Filter Passed Short or Long Pass Filter Band Pass Filter Adsorbed Absorption Filter Electronics Electrical pulses are digitized, the data is stored (‘list mode data’), analysed and displayed through a computer system. The end result is quantitative information about every cell analysed Large numbers of cells can be processed quickly Comprehensive antibody panels The rationale : (1) The lineage of the cells of interest (e.g., myeloid, B-cell, T-cell), (2) Their maturity status, (3) The clonality, where appropriate, (4) The specific subtype of hematopoietic malignancy and (5) The status of the normal elements present. Appropriate isotype controls are included in the panels. The evaluation of the FCM data also relies on internal controls, however (e.g., T-cells serve as internal control for B-cells and vice versa) Abs Panel, the European Group for the Immunological Characterization of Leukemias (EGIL) for the diagnosis and classification of acute leukemia Panel of antibodies recommended by the British Committee for Standards in Haematology (BCSH) for the diagnosis and classification of acute leukemia Panel of antibodies recommended by the European LeukemiaNet, ELN for the diagnosis and classification of acute leukaemia Panel of antibodies recommended by the US– Canadian Consensus Group for the diagnosis and classification of acute leukemia Reactivity of mAbs vs. FAB-AML Development of a FACS histogram 45% Negative cells Counts Positive cells Fluorescent intensity Intensity scales are logarithmic Note: The operator can set the “gate” by visual inspection of the histogram. The “gate” defines negative versus positive. THE OPERATOR SETS “GATES” DEFINING POSITIVE AND NEGATIVE FOR EACH MARKER. CD19 CD19 CD19+ CD3- CD19+ CD3 - CD3 + CD3+ CD3 CD19- CD19- CD3- CD3+ CD3 Therefore, you can define each cell counted with regard to CD19 or CD3 positivity. Note that there are not normally cells in the circulation that express both T and B cell surface markers. Below are the FCM results on a peripheral blood specimen studied) at a teaching hospital: CD2 48% moderate CD3 45% moderate CD4 21% moderate CD7 47% moderate CD8 20% moderate CD13 3% moderate CD33 1% moderate CD34 1% weak TdT 55% moderate CD19 47% moderate CD20 26% moderate CD22 47% moderate sIgM 48% moderate Kappa 3% moderate Lambda 2% moderate CD10 36% moderate CD45 100% strong HLA-DR 55% moderate Interpretation The results indicated a proliferation of immature cells (TdT+). The case was interpreted as ALL with a mixed (B-cell and T-cell) lineage. Because of the data-reporting format, it is unclear whether the immature cells are of B- or T-cell lineage, however. Although fluorescence intensities were mentioned, data interpretation in this particular laboratory was actually based on percent positive with an arbitrary 20% cutoff. When proper visual data analysis was subsequently applied to the raw data, it became apparent that the blood sample contained a clearly identifiable neoplastic population of precursor B-ALL, admixed with a high number of normal T-cells. Steps in Flowcytometry 1. Preanalytical (specimen handling and processing, including antibody staining), 2. Analytical (running the sample through the flow cytometer and acquiring data), and 3. Postanalytical (data analysis and interpretation). Deficiencies such as suboptimal instrument performance, poor reagent quality (antibodies and/or fluorochromes), or poor specimen quality can all result in inadequate resolution of positive and negative immunofluorescence. Preanalytical Phase Little control over certain factors, eg. specimen collection and transportation, which can adversely affect the sample prior to its arrival. Poor specimen collection The time elapsed between specimen acquisition and delivery to the laboratory, and the environmental conditions during transport are critical factors As a rule, specimens cannot be held for more than 48 hours in the fresh state after collection. This time window is much narrower for samples harboring a tumor with a high turnover rate (e.g., Burkitt lymphoma). Exposure to extreme temperatures and the presence of blood clots (or gross hemolysis) are conditions that can render a blood or bone marrow specimen unacceptable for analysis. Fresh specimens for FCM Liquid samples (peripheral blood, bone marrow, body fluids) and Solid tissue (lymph nodes, tonsils/ adenoids, spleen, bone marrow biopsies, and extranodal infiltrates). Specimen Type PB & BMA can be collected in either EDTA or heparin. The volume required depends on the WBC count; 10 mL of blood is adequate in most instances. Store & transfer at RT (at RT in delay). Referred blood ,should be accompanied by a hemogram and a fresh blood smear Approximately 3 to 5 mL of BMA is usually sufficient for a comprehensive FCM analysis, Degenerative changes in BMA tend to occur more quickly than PB Diagram of lymph node slicing and the allocation of the slices to different studies F, FCM analysis; H, histology; I, immunohistochemistry; M, molecular studies. Each slice is less than 2 mm thick. Preparing nucleated cell suspensions Cell yield and viability Sample staining - Surface antigens, staining is performed on viable unfixed cells. All staining is performed at 40C to minimize capping and antigen shedding. Appropriate isotype controls are included. The usual number of cells recommended for immunostaining is 106 cells (low cell yield, it is possible to perform the staining with as few as 1 × 105 to 2 × 105 cells/tube) - Intracellular antigens, the staining procedure is more laborious than cell surface antigen staining and calls for cell fixation and permeabilization Case 0f ALL Immunophenotype: Immunophenotyping of Bone marrow aspirate by flow cytometry shows predominant a B cell population (about 89% of the cells analyzed) The majority of these B cells show expression of CD10, CD19, HLA-DR. They were negative for CD2, CD5, CD7, CD13, CD33, CD20 and Tdt. Review of BMA smear shows a predominant lymphoblast population (65%). Dual Positive for CD10 / CD19. Cytochemistry: Myeloperoxidase: All leukemic blasts were MPX negative. Interpretation / Diagnosis: Immunophenotyping results, together with morphological findings and Cytochemistry of BMA, are consistent with B lymphoblastic leukemia (Early pre B-cell type). AML-M3 , Classic or Hypergranular type Immunophenotype: Immunophenotyping of BMA by flow cytometry shows a predominant leukemic cell population (about 85% of the cells analyzed, Gated on region 1) that is positive for CD13, CD33 & CD117,CD45. The majority of gated cells were negative for CD2,CD3,CD4,CD5,CD7,CD8,CD10, CD11b, CD11c ,CD14,CD19, CD20,CD25,CD41, CD61, HLA-DR. These cells have intermediate granularity (based on side-scatter signal). Cytochemistry: All of the leukemic cells were intensely myeloperoxidase Positive. File: CD10.FCS Date: 09-11-2011 Time: 13:40:32 Immunophenotyping results, together 400 with morphological Gate: R1 findings and Cytochemistry of BMA, are consistent with B lymphoblastic leukemia (Early 320 pre B-cell type). RN1 200 File: CD19.FCS Date: 09-11-2011 Time: 13:42:24 Particles: 13036 Acq.-Time: 57 s 200 160 SSC - 250150 200 100 Gate: R1 RN1 160 R1 counts 120 150 SSC - partec PAS 240 counts 250 50 100 50 Particles: 13134 Acq.-Time: 48 s R1 0 0 50 80 80 0 40 100 150 FSC - 200 250 1 10 100 FL1 CD10 1000 0400 0 1000 0 50 100 150 200 250 1 10 100 1000 CD34.FCS File: HLA.FCS Date: Time: 13:54:48 File: Particles: 13551Date: Acq.-Time: 38 s Time: 13:56:48 Particles: 14047 Acq.-Time: 12 s FSC 09-11-2011 FL109-11-2011 200 1000 Gate: R1 200 200 250 250 Gate: R1 Gate: R1 RN2 RN2 FL2 CD19 SSC counts counts counts SSC - R1 120 150 80 10 100 50 0 1 1 10 100 1000 FL2 CD19 0 50 100 150 200 250 0 Gate FSCCount Region Ungated Count/ml 200 1 Gate: R110674 10 10674 R1 <None> - 100 FL2 RN1 R1 93 47 - 0 RN1 160 120 R1 80 10 40 80 100 100 120240 150 160100 RN1 160 200 200 FL2 - 160 counts 320 80 partec PAS 50 40 40 1 00 0 10 100 1000 0 50 100 - 150 200 250 FL1 1 10 100 1000 FSC1FL1 HLA 100 %Gated Mean-x CV-x% Mean-y 1000 Gate: R1 1000 R1 81.88 Gate: 72.44 16.39 1 39.74 0.44 Q1: 3.33% 2.96 22.79 Q2: 22.52% - 1 10 100 FL1 CD34 1000 CV-y% Gate: R1 100 10 31.43 0.00% FL1Q1:CD10 - 1000 1000 Q2: 0.14% Patient Report Format Patient Report-2 Format Hematogone vs.Leukemic Lymphoblast Diagnosing Multiple Myeloma Three Diagnostic Criteria Required for a Positive Diagnosis of Multiple Myeloma 1 • Monoclonal plasma cells present in the bone marrow ≥10% • Presence of a documented plasmacytoma 2 • Presence of M component in serum and/or urine* • One or more of the following (CRAB criteria): 3 Calcium elevation (serum calcium >11.5 mg/dL) Renal insufficiency (serum creatinine >2 mg/dL) Anemia (hemoglobin <10 g/dL or 2 g/dL <normal) Bone disease (lytic lesions or osteopenia) *Monoclonal M spike on electrophoresis IgG >3.5 g/dL, IgA >2 g/dL, light chain >1 g/dL in 24-hour urine sample. Durie et al for the International Myeloma Working Group. Leukemia. 2006:1-7. 53 Diagnostic Evaluation of Multiple Myeloma Test Finding(s) With Myeloma CBC with differential counts ↓ Hgb, ↓ WBC, ↓ platelets Electrolytes ↑ Creat, ↑ Ca+, ↑ Uric acid, ↓ Alb Serum electrophoresis with quantitative immunoglobulins ↑ M protein in serum, may have ↓ levels of normal antibodies Immunofixation Identifies light/heavy chain types M protein β2-microglobulin ↑ Levels (measure of tumor burden) C-reactive protein ↑ Levels (marker for myeloma growth factor) 24-hour urine protein electrophoresis ↑ Monoclonal protein (Bence Jones) Bone marrow biopsy ≥10% plasma cells Skeletal imaging Osteolytic lesions, osteoporosis Serum free light chain ↑ Free light chains MRI Evaluation of involvement of disease Alb = albumin; CBC = complete blood count; Creat = creatinine; Hgb = hemoglobin; MRI = magnetic resonance imaging; WBC = white blood cell Abella. Oncology News International. 2007;16:27; Barlogie et al. In: Williams Hematology. 7th ed. 2006:1501; Durie et al. Hematol J. 2003;4:379; MMRF. 54 Multiple Myeloma: Disease Overview. 2006. www.multiplemyeloma.org; Rajkumar et al. Blood. 2005;106(3):812. Peripheral blood - rouleaux B.Poopak Malignant Plasma Cells in Marrow Myeloma: A Cancer of Plasma Cells in the Bone Marrow Patients with multiple myeloma show a "spike" in special regions of the serum protein electrophoresis Serum Protein Electrophoresis Normal Monoclonal Protein in Myeloma Kyle RA and Rajkumar SV. Cecil Textbook of Medicine, 22nd Edition, 2004 Figure 8. Quantitative immunoglobulins were within normal limits Maslak, P. ASH Image Bank 2001;2001:100211 Copyright ©2001 American Society of Hematology. Copyright restrictions may apply. Figure 8. Immunofixation electrophoresis showing a monoclonal IgA lambda light chain restricted band Lazarchick, J. ASH Image Bank 2001;2001:100185 Copyright ©2001 American Society of Hematology. Copyright restrictions may apply. Immunofixation to Determine Type of Monoclonal Protein IgG kappa M protein Lambda Light Chains Kyle RA and Rajkumar SV. Cecil Textbook of Medicine, 22nd Edition, 2004 CASE STUDY – MULTIPLE MYELOMA Serum free light chains Free kappa Free lambda Ratio 16.2 3754.1 0.0 3.3 – 19.4 5.7 – 26.3 0.3 – 1.7 mg/L mg/L Interpretation Free lambda light chain monoclonal gammopathy Radiology Diffuse osteolytic lesions in thoracic and lumbar regions with several compression fracturres Normal serum Immunofixation Report: Polyclonal Pattern serum Immunofixation Report: Mono-Clonal IgA – Kappa Pattern serum Immunofixation Report: Mono-Clonal IgM – Kappa Pattern Thank you, any question?