Introduction to Flow Cytometry
advertisement

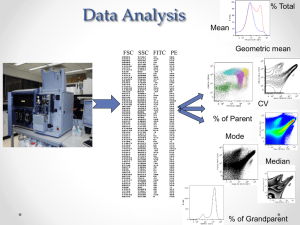
Introduction to Flow Cytometry IGC Workshop Fundamentals of Flow Cytometry (cont.) IGC – April 27, 2010 The Instrument 2 Filters Blocked FilteredFilteredBlocked BP LP::Band Long Pass Pass Filter Filter 530> /500 60 4 Filters 5 Optical Layout 6 Detection PMT Photo Multiplier Tube PMT’s collect photons that are then converted into voltage signals 8 Pulse Flowing Stream Voltage pulse Laser 9 Pulse Parameters W H H: A A: W: 10 Height vs Area H A H A For non spherical cells, Height (FL-H) is not an adequate parameter to analyze Area (FL-A) is the most adequate. However, we still need to remove doublets from the analysis... 11 Doublet Discrimination W 2W W 2H H 2A doublets 2A FL-H A FL-W H Single cells FL-A FL-A 12 Threshold Forward Scatter Threshold H W Threshold Time Small Cells and debris Cells of Interest 13 Analysis Software Flowjo VenturiOne CellQuest Summit FCSExpress Kaluza FACSDiva 15 Histograms 9574 Cell Count Counts 7180 4787 2393 0 100 101 102 FL1 Log Fluorescence Fluorescence in a cell 103 104 What are those dots? Gating Common Gate Shapes Logical Gating AND, OR, NOT 18 Gating Positive or Negative? A “positive” cell or event is that which falls outside the “negative” gate. Neg Pos 19 Back Gating Back gating a positive population can enrich the population of interest and help identify it correctly CD4 FITC 20 Acquisition How many cells should I acquire? Counting cells follows Poisson statistics: cv % = 1 Precision cv % = sd x 100 mean x 100 N N is the number of cells counted Example: Population of interest is 1% of total population and want 5% precision 1002 10,000 N= = = 400 (cv %)2 25 40,000 Number of cells to be counted in the region of interest Number of total cells to be counted 21 Dot vs Countour Plots Dot Plots Contour Plots 22 Logarithmic or Linear? Anti-CD4-labeled antibody Signals vary >100-fold Use Log scale Linear Log DNA-labeling dye Signals vary 2- to 10-fold Use Linear scale Linear Log 23 Introduction to Flow Cytometry IGC Workshop Fundamentals of Flow Cytometry (end) IGC – April 27, 2010