SEMESTER TEST 01 (10-03-2012)
advertisement

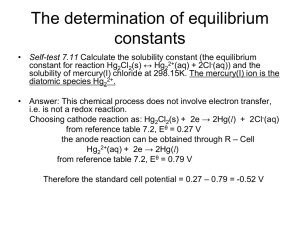
QWAQWA CAMPUS / QWAQWA KAMPUS CEM 214 UNIVERSITEIT VAN DIE VRYSTAAT UNIVERSITY OF THE FREE STATE SEMESTER TEST 1 ASSESSOR: Sat, 10 March 2012 Ms. C.E. Clarke MODERATOR: Prof. A.S. Luyt TIME : 1 Hour MARKS: 50 ANSWER ALL QUESTIONS AND SHOW ALL CALCULATIONS WITH AN INK PEN -- NO PENCILS OR "TIPPEX" ALLOWED / PAPER CONSISTS OF PAGE(S) AND QUESTIONS. RECORD ALL ANSWERS UP TO TWO (2) SIGNIFICANT FIGURES. THERMODYNAMICS / 40 marks: 1. Estimate the molar combustion enthalpy of ethanol, CH3CH2OH(l), by using the appropriate average bond energies data. Assume that the evaporation enthalpy of CH3CH2OH(l) = 47 kJ mol-1 and that of H2O(l) = 31 kJ mol-1. Write the equation of the combustion of ethanol. Use T = 298 K and show all steps. [10] Bond Energy /kJ mol-1 C-C 343 O-H 464 C=C 615 O-O 144 CC 812 O=O (in O2) 498 C-O 351 H-H 436 C=O 724 C-H 416 C=O (in CO2) 799 2. Derive from the Second Law of Thermodynamics an equation in terms of a, b, c and T to calculate the entropy change, S, of water vapour as a function of temperature in a closed system during a reversible, isobaric temperature change. Given Cp(H2O) (g) = a + bT + cT-2 [7] 3. One mole ideal gas undergoes a reversible isothermal expansion until its volume is doubled. If the gas performed 1 kJ work on the surroundings, what is the temperature? [4] 4. The densities of ice and water at 0C are 0.9168 g.cm-3 and 0.9998 g.cm3, respectively. If H for the fusion process at atmospheric pressure is 6.025 kJ.mol-1, what is U? How much work is done on the system? [15] 5. One mole of an ideal gas, with CV,m = 3/2R, is heated from 298 K to 353 K, at constant pressure. Calculate S for the system. [4] MEMORANDUM: 1. 10 marks CH3CH2OH(l) + 3O2(g) 2CO2(g) + 3H2O(g) ∆Ug [BE(C-C) + 5xBE(C-H) + BE(C-O) + BE(O-H) + 3xBE(O=O)] – [4xBE(C=O) + 6xBE(H-O)] ∆Ug [343 + 5x416 + 351 + 464 + 3x498] kJ mol-1 - [4x799 + 6x464] kJ mol-1 ∆Ug [4732 – 5980] kJ mol-1 = -1248 kJ mol-1 ∆Hg = ∆Ug + ∆(PV) where ∆(PV) is small ∆Hg ∆Ug = -1248 kJ mol-1 -1248 kJ mol-1 CH3CH2OH(g) + 3O2(g) 2CO2(g) + 3H2O(g) 47 kJ mol-1 CH3CH2OH(l) CH3CH2OH(g) 3H2O(g) 3H2O(l) -93 kJ mol-1 CH3CH2OH(l) + 3O2(g) 2CO2(g) + 3H2O(l) -1294 kJ mol-1 2. 7 marks S dq p T T2 C p dT S T T1 dH T and dH C p dT a bT cT 2 dT T T1 T2 T2 T2 c a S b cT 3 dT S a ln T bT T 2T 2 T1 T1 S a ln T2 bT2 T1 T1 c 1 1 2 2 2 T2 T1 3. 4 marks dw - PdV dw - (nRT/V)dV dw - nRT - w -nRTln V2 1 VdV V1 V2 V1 - 1000J - (1 mol)(8.314 J.K - 1.mol - 1)T ln 1000 J - (1 mol)(8.314 J.K - 1.mol - 1)ln2 173.5K T 2 1 4. 15 marks Mr(H2O) = 2(1.00) + (16.0)g.mol-1 = 18.01 g.mol-1 1 mol of ice has a MASS (m) = Mr(H2O) x n = (18.01 g.mol-1) x (1 mol) = 18.01 g 1 mol of water has a MASS (m) = Mr(H2O) x n = (18.01 g.mol-1) x (1 mol) = 18.01 g 1 mol of ICE has a VOLUME (Vice) = m/ = (18.01 g) / (0.9168 g.cm-3) = 19.64 cm3 1 mol of WATER has a VOLUME (Vwater) = m/ = (18.01 g) / (0.9998 g.cm-3) = 18.01 cm3 V(water ice) = (18.01 – 19.64) cm3mol-1 = -1.63 cm3.mol-1 = -0.00163 dm3.mol-1 (PV) = -0.00163 dm3.mol-1 x 1 atm = -0.00163 dm3.mol-1 x 101.325 J = - 0.165 J.mol-1 H = U + PV = 6.025kJ = 6025 J 6025 J = U + (-0.165 J.mol-1) U = 6024.84 J.mol-1 = 6025 J.mol-1 = 6.025 kJ.mol-1 Work done on the system = 0.165 J.mol-1 5. 4 marks CV,m = 3/2R CP,m = 3/2R + R = 5/2R 353 Sm = ∫298 (CP,m)dT/T = 5/2R ln(T2/T1) = 5/2 x (8.3145 J.K-1.mol-1) x ln(353/298) = 3.52 J.K-1.mol-1