Introduction to Second Law (contd.) [Lecture 4].
advertisement
![Introduction to Second Law (contd.) [Lecture 4].](http://s2.studylib.net/store/data/005767600_1-250da23339ac97bb85a82c10f66d9a9f-768x994.png)
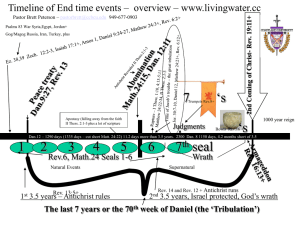
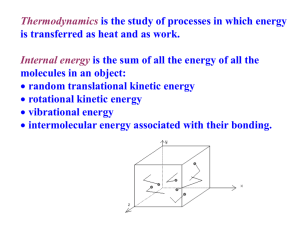
MEL140 (Second Law: Continued) Recap on Irreversible process • A system undergoes an irreversible process, when – second law demands that reversal of the process leaves a finite trace on the surroundings. Connection with the Kelvin-Planck statement (board). OR equivalently. – Traceless reversal is prohibited by second law (Connection with Clausius statement) Ways to look at “irreversibilities”: Lack of thermodynamic equilibrium between system and surroundings renders (existence of “driving forces”) a process irreversible: Lack of thermal equilibrium: finite temperature differences. Lack of mechanical equilibrium: finite pressure differences (e.g. free expansion). Chemical equilibrium: e.g. reactions/phase-transformations that complete, diffusion of dye/ink in water. General sign of lack of equilibrium: if the system is isolated and observed instantaneously, processes (internal adjustments) will be found to occur. Recap on Irreversible process Ways to look at “irreversibilities”: General sign of lack of equilibrium: if the system is isolated and observed instantaneously, processes (internal adjustments) will be found to occur. During an irreversible process, “internal currents/fluxes” that lead to dissipation are present due to driving forces either between the system and surroundings or between parts of a system. Dissipation can be identified when during a process without thermal interaction with the surroundings, the sum of the macroscopic potential and macroscopic kinetic energy of the system decreases. (energy goes to “microscopic modes”, remember discussion on work energy theorem) e.g. mechanical friction, shocks/”explosions”, plastic deformations, “Joule heating effects” (resistors differs from capacitor/inductor), eddy currents. Moving from one equilibrium state to another is possible in finite time only by irreversible (fast) processes. To show that heat transfer through a finite temperature difference is an irreversible process tH tH Q1-Q Q1 W=Q1-Q H Q W=Q1-Q Q tC Violation of Kelvin Planck statement Example of irreversibility due to lack of equilibrium: unrestrained expansion of a gas A 800 kPa B 0 kPa A membrane separates a gas in chamber A from vacuum in chamber B. The membrane is ruptured and the gas expands Into chamber B until pressure equilibrium is established. The process is so fast and the container is insulated enough such that negligible heat transfer takes place between the gas and the surroundings during this process. At the end of the unrestrained expansion process, the gas (system) has the same internal energy, as it had initially. Some questions not yet answered • What kind of engines and refrigerators have the best possible performance? • What factor(s) affect the performance of a heat engine and a refrigerator? • What is the best possible performance of a heat engine and a refrigerator? The Carnot principles • First Carnot principle: The efficiency of an irreversible heat engine is always less than the efficiency of a reversible heat engine operating between the same two reservoirs. (Irr<rev) • Second Carnot principle: All reversible engines operating between the same two reservoirs have the same efficiency. Rev1 =Rev2 η= W ork delivered H eat input from the hot reservoir W net , out QH Proof of First Carnot principle • Proof by contradiction: Assume Irr>Rev η= W net QH tH Q Q Wirrev-Wrev WRev <Wirrirr W <W Rev WIrr=Q-QIrr Rev+Irr Rev Irr QIrr QRev >QIrr Q Rev>Q Irr TtLC QRev-QIrr tL Conclusion: Assumption Irr>Rev is incorrect. Efficiency of a reversible engine is higher than that of an irreversible engine. Proof of Second Carnot principle • Proof by contradiction: Assume Rev1>Rev2. tH QQ Q WRev1-WRev2 W WRev2 <WRev1 Rev2<W Rev1 WRev1=Q-QRev1 Rev1+Rev2 Rev2 Rev2 Rev1 QRev1 QRev2 >QRev1 Q Rev2>Q Rev1 TtLC Conclusion so far: Assumption Rev1>Rev2 is incorrect. QRev2-QRev1 tL Rev 1 Rev 2 Proof of Second Carnot principle (continued) • Proof by contradiction (continued): Assume Rev1<Rev2. tH QQ Q WW <W<W Rev1 Rev2 Rev1 Rev2 WRev2-WRev1 WRev2=Q-QRev2 Rev1+Rev2 Rev2 Rev1 Rev1 QRev1>Q Q Rev1 Rev1 Tt C L QRev2 QRev1-QRev2 tL Final conclusion: Rev1=Rev2. All heat engines working between the same reservoirs have the same efficiency. An important implication of the second Carnot principle • The efficiency of a reversible heat engine does not depend on its working fluid, method of execution of cycle, type of reversible engine used, amount of heat drawn from or rejected by the engine etc. It may however depend on a characteristic of the reservoirs. • By what characteristic is a reservoir specified? Ans.: Temperature. • The only factors that could affect the efficiency of a reversible engine is, therefore, the temperatures (tH,tL) of the reservoirs it is connected to. rev f ( t H , t L ) Uses of the second Carnot principle • To develop a thermodynamic temperature scale, which is a temperature scale that does not depend on the properties of a particular substance. • To calculate the maximum efficiency of a heat engine (or maximum COP of a refrigerator/heat pump). Empirical and thermodynamic temperature scales Empirical temperature scale Thermodynamic temparature scale • • • • A scale that is based on the measurement of a temperaturesensitive property of a certain substance (e.g. pressure exerted by a constant volume of helium gas, thermal expansion of a enclosed mass of mercury/alcohol etc.). A thermometer reads the “empirical temperature”. Notation for empirical temperature: (t) A scale that is independent of the properties of any substance. • Lord Kelvin was aware of the second Carnot principle and suggested in1848, that a thermodynamic temperature scale could be based on the theoretical consideration that, during the operation of a reversible heat engine, the amounts of heat exchanged between system and the reservoirs depend only on the temperature of the reservoirs and not on the properties of any substance. Notation for the thermodynamic temperature scale to be developed : (T) Empirical temperature scales (t) • Empirical scales are determined through experimentation with “thermometric substances”. • Single fixed point scale (>1954) • Constant volume gas thermometer and the ideal gas temperature scale. t ideal gas 273.16 p p tp The ideal gas temperature scale: a very accurate empirical temperature scale developed from experiments using the constant volume gas thermometer • Step 1: Bring the thermometer in contact with water at triple point (tp). Measure the pressure ptp. • Step 2: Bring the thermometer in contact with the body at temperature T. Measure the pressure p. Calculate t m easured 273.16 p p tp • Now, redo steps 1 and 2, at each instance reducing the number of moles (mass) of gas used in step 1 (and 2), such that pt=2n (n=10,9,8,.,3 etc.) mm Hg. • Perform this experiment with various gases (A,B,C) • C Capillary tube L Lip M Mercury manometer Gas A Gas B Gas C tmeasured At low pressures, ideal gas behavior is approached by all gases. ptp A temperature scale based on the second Carnot principle • Since rev ( t H , t L ) But rev ( t H , t L ) W net , out QH QL QH QL QH f (t H , t L ) QH 1 QL QH QH f (t , t Q Properties of according to second law H L ) L f ( t1 , t 3 ) f ( t1 , t 2 ) f ( t 2 , t 3 ) Q1 Q2 Q2 Q3 f ( t1 , t 2 ) f ( t1 , t 2 ) f (t 2 , t3 ) Q1 Q3 Q3 f (t 2 , t3 ) f ( t1 , t 3 ) QH T (t H ) T (t L ) Q L rev T ( t1 ) T (t 2 ) T (t 2 ) T (t3 ) Constructing a single-fixed-point thermodynamic temperature scale • Place one of the reservoirs Q1 T ( t 2 ) T ( t1 ) Q 2 rev in thermal equilibrium with water at triple point (tp); prescribe the value 273.16 to the constant T ( t1 ) Procedure for calculating thermodynamic temperature for t>ttp for t<ttp The reversible engine is operated with a fixed Qtp ; then Q(t) is measured to obtain T(t) using: Q (t ) T ( t ) 273.16 Q t p r ev T is known as the Kelvin/absolute temperature scale (in either case) The Kelvin scale is not the only thermodynamic temperature scale. • The Kelvin scale calculates the thermodynamic temperature using : Q (t ) T ( t ) 273.16 Q t p r ev ( Q ( t ) T ( t ) T '( t ) ) Alternatively any monotonic function T’(t) of T(t) can also be chosen to define a new thermodynamic temperature scale. T (t ) 2 e . g . T ( t ) , T ( t ) 273.15, ln 27 3.16



![Introduction to Second Law (contd.) [Lecture 5].](http://s2.studylib.net/store/data/005616309_1-e04677ea698eaf2815262e3c7bbb995c-300x300.png)