File - El Paso High School
advertisement
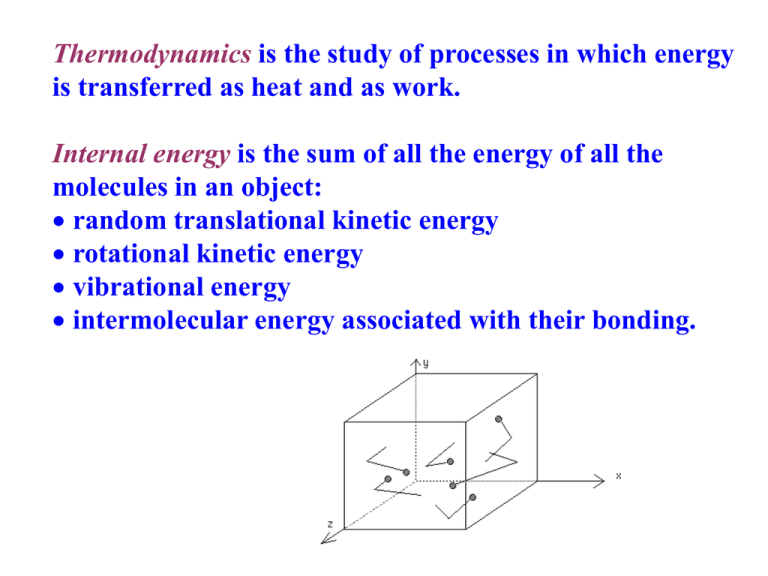
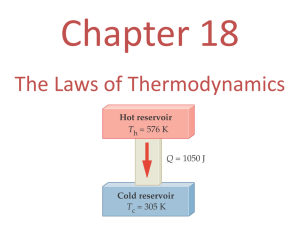
Thermodynamics is the study of processes in which energy is transferred as heat and as work. Internal energy is the sum of all the energy of all the molecules in an object: random translational kinetic energy rotational kinetic energy vibrational energy intermolecular energy associated with their bonding. ZEROTH LAW The Zeroth law states that "If bodies A and B are each separately in thermal equilibrium with body C, then A and B are in thermal equilibrium with each other." The common property between A and B is called temperature. FIRST LAW OF THERMODYNAMICS ΔU = Q + W In any thermodynamic process, the change in internal energy (ΔU) is equal to the net heat (Q) absorbed by a system plus the work (W) done on the system. Q is (+) if added and (-) if removed W (-) if done by the system and (+) if done on the system. What does it mean for the system to do work? Work is simply a force multiplied by the distance moved in the direction of the force. A good example of a thermodynamic system that can do work is the gas confined by a piston in a cylinder, as shown in the diagram. PRESSURE-VOLUME (PV) DIAGRAMS Many thermodynamic processes involve energy changes that occur to gases enclosed in cylinders. The work done by the gas when it expands at a constant P is shown in the figure: When a thermodynamic process involves changes in volume and/or pressure, the work done by the system is equal to the area under the curve of a PV diagram. When the gas suffers changes which eventually return it to its initial state, it is said to have undergone a cycle of operations and the diagram is a closed loop. The net work done by the gas in this case is represented by the enclosed area. THERMODYNAMIC PROCESSES There are a number of different thermodynamic processes that can change the pressure and/or the volume and/or the temperature of a system. TYPES OF THERMODYNAMIC PROCESSES: • Adiabatic • Isovolumetric • Isothermal • Isobaric ISOVOLUMETRIC PROCESS An isovolumetric (or isochoric) process is one in which the volume of the system remains constant. ΔW = 0 so: ΔQ = ΔU All the thermal energy absorbed by a system goes to increase its internal energy, and there is a rise in the temperature of the system. ISOTHERMAL PROCESS An isothermal process is one in which the temperature of the system remains constant. ΔU = 0 so: -ΔQ = ΔW All the energy absorbed by a system is converted into output work. ISOBARIC PROCESS In an isobaric process the pressure remains constant and of the heat received by the gas, some becomes internal energy as the temperature rises, the rest is used to do work. ΔU = Q + W ADIABATIC PROCESS An adiabatic process is one in which there is no exchange of thermal energy ΔQ between a system and its surroundings. ΔQ = 0 so: ΔW = ΔU In an adiabatic process the work is done at the expense of internal energy. The decrease in internal energy is usually accompanied by a decrease in temperature. - An example of an isobaric system is a gas, being slowly heated or cooled, confined by a piston in a cylinder. - An example of an isovolumetric system is a gas in a box with fixed walls. - An example of an isothermal system is a gas confined by a piston in a cylinder where the gas is not heated or cooled, but the piston is slowly moved so that the gas expands or is compressed. The temperature is maintained at a constant value by putting the system in contact with a constant-temperature reservoir (the thermodynamic definition of a reservoir is something large enough that it can transfer heat into or out of a system without changing temperature). - An example of an adiabatic process is a gas expanding so quickly that no heat can be transferred. The expansion does work, and the temperature drops. This is exactly what happens with a carbon dioxide fire extinguisher, with the gas coming out at high pressure and cooling as it expands at atmospheric pressure. SECOND LAW OF THERMODYNAMICS The second law of thermodynamics can be stated in several equivalent ways, three of which are: 1. Heat energy flows spontaneously from a hot object to a cold object only. 2. It is impossible to construct a heat engine that is 100% efficient. A heat engine can convert some of the input heat into useful work, but the rest must be exhausted as waste heat. 3. The entropy of an isolated system never decreases. It can only stay the same or increase. First Statement of the Second Law The first statement of the second law is a statement from common experience. When two objects, one hot and the other cold, come into contact, heat energy will be transferred from the system at higher temperature to the system at lower temperature, but not vice versa. While the first law states that energy must be conserved, i.e., the sum of the energy lost and gained in any process must equal zero, it does not say that heat must flow from the hot object to the cold object. The first law would not be violated if the hot object became hotter while the cold object became colder. The second law states that the direction of the heat flow must be from hot to cold. Second Statement of the Second Law: Heat Engines A heat engine is a device that is capable of changing thermal energy (QH), also known as the input heat or heat of combustion of the fuel, into useful work (W). Heat engines cannot be made to be 100% efficient and while part of the heat energy is converted to useful work, the remaining heat energy will be rejected to the environment or surroundings as waste heat (QL) like the exhaust from a car engine for example. Therefore, Q H = W + QL CARNOT ENGINE In an idealized engine, known as a Carnot engine, energy losses due to internal friction or turbulence present in the fuel after ignition, etc. are not considered. The most efficient heat engine cycle is the Carnot cycle, consisting of two isothermals and two adiabatic processes. The Carnot cycle can be thought of as the most efficient heat engine cycle allowed by physical laws. When the second law of thermodynamics states that not all the supplied heat in a heat engine can be used to do work, the Carnot efficiency sets the limiting value on the fraction of the heat which can be so used. Carnot determined that the maximum efficiency (e) that could be realized from a heat engine depends on the temperature of the input heat (TH) and the exhaust heat (TC) where TH and TC are expressed in Kelvin. The maximum efficiency (e) or Carnot efficiency of a heat engine is determined by: (QH QL ) W but since W = QH - QL then e e QH QH Using the input and waste heat temperatures: (TH TL ) e TH In order for a Carnot engine to be 100% efficient, it would be necessary for the temperature of the exhaust heat to be at absolute zero (zero Kelvin). This is a practical as well as a theoretical impossibility. Refrigerators and Air Conditioners Refrigerators and air conditioners operate by removing heat from a low temperature (cold) reservoir and exhausting the heat to the higher temperature (hot) reservoir. In order to accomplish this task, work is done to cause heat to travel opposite from its normal direction. 15.1 In a certain process, 8.00 kcal of heat is supplied to the system while the system does 6.00 kJ of work. By how much does the internal energy of the system change during the process? ΔQ = 8000 cal (4.186) = 33,488 J = 33.5 kJ ΔW = - 6 kJ ΔU = ΔQ + ΔW = 33.5 - 6 = 27.5 kJ 15.2 The specific heat of water is 4180 J/kg.K. By how many joules does the internal energy of 50 g of water change if it is heated from 21°C to 37°C? c = 4180 J/kg.K m = 0.05 kg to = 21°C tf = 37°C ΔQ = mc ΔT = 0.05 (4180)(37 - 21) = 3344 J since ΔW = 0 ΔU = ΔQ = 3344 J 15.3 Find the change in work and the change in internal energy for a 1700 g cube of iron as it is heated from 20 °C to 300 °C at atmospheric pressure. For iron, c = 0.11 cal/g°C and the change in volume is 2.18x10-6 m3. c = 0.11 cal/g°C m = 1700 g to = 20°C tf = 300°C ΔV = 2.18x10-6 m3 Patm = 1x105 Pa ΔQ = mc ΔT = 1700 (0.11)(300 - 20) = 52,360 cal ΔW = P ΔV = 1x105 (2.18x10-6) = - 0.218 J (negative since its done BY the system) ΔU = ΔQ - ΔW = 52,360 cal (4.186 J/cal) - 0.218 = 2.19x105 J 15.4 In each of the following situations, find the change in internal energy of the system a. A system absorbs 500 cal of heat and at the same time does 400 J of work. Q = 500 cal W = - 400 J ΔU = ΔQ - ΔW = 500 (4.186) – 400 = 1693 J = 1.69 kJ b. A system absorbs 300 cal of heat and at the same time 420 J of work is done on it. Q = 300 cal W = 420 J ΔU = ΔQ + ΔW = 300 (4.186) + 420 = 1675.8 J = 1.67 kJ c. 1200 cal are removed from a gas held at constant volume. Q = - 1200 cal ΔU = ΔQ + ΔW = - 1200 (4.186) + 0 = - 5023.2 J = -5.02 kJ 15.5 For each of the following adiabatic processes, find the change in internal energy. a. A gas does 5 J of work while expanding adiabatically. W=-5J Adiabatic ΔQ = 0 ΔU = ΔQ - ΔW =0-5=-5J b. During an adiabatic compression, 80 J of work is done on a gas. W = 80 J ΔU = ΔQ + ΔW = 0 + 80 = 80 J 15.6 1 kg of steam at 100°C and 101 kPa occupies 1.68 m3. a. What fraction of the observed heat of vaporization of water is accounted for by the expansion of water into steam? m = 1 kg, t = 100°C P = 101 kPa, V = 1.68 m3 Lv = 2.256x106 J/kg ΔW = PΔV = 101x103 (1.68) = 169,680 J ΔQ = mLv = 1 (2.256x106) = 2.256x106 J Fraction: ΔW/ ΔQ = 169,680/2.256x106 = 0.075 = 75% b. Determine the increase in internal energy of 1.0-kg of water as it is vaporized at 100 °C. m = 1 kg t = 100°C W = - 169,680 J ΔU = ΔQ - ΔW = 2.256x106 - 169,680 = 2.08x106 J 15.7 The PV diagram in the figure applies to a gas undergoing a cyclic change in a piston-cylinder arrangement. What is the work done by the gas: a. In portion AB of the cycle? Work = area under AB W = (4 - 1.5)x106 (4x105) =1J b. In portion BC? ΔV = 0 therefore W = 0 J c. In portion CD? ΔV (-) (contraction) W = - (2.5x10-6)(2x105) = -0.5 J d. In portion DA? W=0J e. The net work output of the gas during the cycle ΔW = 1 - 0.5 = 0.5 J or area enclosed: ΔW = (2.5x10-6)(2x105) = 0.5 J f. The net heat flow into the gas per cycle. ΔQ = ΔU + ΔW ΔU = 0 ΔQ = 0 + 0.5 = 0.5 J 15.8 For the thermodynamic cycle shown in the figure, find the net work during the cycle. Area of the figure = Net Work Area ≈ 22 squares A = (0.5x105) (0.1)(22) = 110,000 J ΔW = 110 kJ 15.9 A steam engine operating between a boiler temperature of 220 °C and a condenser temperature of 35 °C delivers 8.0 hp. If its efficiency is 30% of that of a Carnot engine operating between these temperature limits, a. How many calories are absorbed each second by the boiler? eff = 30% Tc = 35 + 273 = 308 K, Th = 220 + 273 = 493 K P = 8 hp (746 W/hp) = 5968 W 5968 J/s (1 cal/s)/4.186 J = 1425.7 cal/s Tc actual eff = 0.3 Carnot eff 0.3(1 ) Th 308 eff 0.3(1 ) = 0.113 493 Wout Qin eff 1425.7 = 12616 cal/s = 12.6 kcal/s 0.113 b. How many calories are exhausted to the condenser each second? Qin = Wout + Qout Qout/s = Qin/s - Wout/s Wout = Qin (eff) Qout/s = Qin/s (1 - eff) = 12.6 (1 - 0.113) = 11.17 kcal/s