CLAUSIUS-CLAPERYON EQUATION I. Review The exact
advertisement

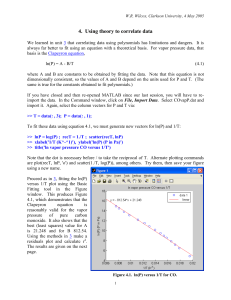
CLAUSIUS-CLAPERYON EQUATION I. Review The exact Clapeyron equation, dp / dT S / V , was obtained for two-phase equilibrium in a one-component system. II. Lecture topics *A. Clapeyron Equation for solid-liquid and solid-solid equilibria *B. Approximate Clausius-Clapeyron Equation for liquid-gas and solid-gas d ln p / d (1/ T ) H / R C. Effect of hydrostatic pressureon vapor pressure A. Clapeyron Equation for Condensed Phases By choosing b to be the high-temperature phase, DH is always positive and the slope of the coexistence line depends on the sign of DV. For melting, most DV = Vliq –Vsol > 0 but in a few cases (H2O, Ga, Ge, Si, Bi) DV < 0. In many cases of solid-solid or solid-liquid, the coexistence line is approximately linear B. Vaporization and Sublimation For X(l) = X(g) [or X(s) = X(g)], one always has DH > 0 and DV > 0, and the coexistence line is curved. We can rewrite the exact Clapeyron equation in the form Where Z Z g Zl pV / RT is the difference in compressibility factors for gas and liquid. Empirically one finds that a plot of ln p vs. 1/T is close to linear. In a range of low pressures and temperatures (far from the critical point), one can obtain H from the slope of ln p vs. 1/T: If we go one step further and 3. assume DH is independent of T, it is possible to integrate Eq. (4) to obtain Trouton's Rule is an empirical rule that works roughly (to within ±10%) for a wide range of normal liquids. Tb is the boiling point of the liquid at p = 1 atm. Trouton's rule can be rationalized on the basis of the ratio of the "free volumes" of the gas (ca. 30,000 3 -1 3 -1 cm mole ) and the liquid (ca. 10% of Vliq, or 3 cm mole ). From statistical mechanics (5.62), close to the empirical value ! C. Effect of inert gas pressure on the vapor pressure p of liquid A At constant temperature T: po is vapor pressure of A in the absence of inert gas P = p + pinert is total pressure if inert gas present at partial pressure pinert At equilibrium μA(g,T,p) = μA(l,T,P) and where the gas phase is treated as a mixture of ideal gases. One can rewrite Eq. (7a) as and integrate to obtain This effect is small in practice. For example, po = 0.27 Torr for Hg at 100°C. The vapor pressure is p = 0.2701 Torr when P = 1 bar and p = 0.283 Torr when P = 100 bar. D. Sample Problem –3 Liquid water has a triple point at 6.11 x 10 bar and 273.16K and its normal boiling point is 1.013 bar (= 1 atm) and 373.15K. The latent heat of vaporization H vap varies –1 –1 with temperature, ranging from 45,050 J mol at ~273K to 40,660 J mol at ~373K. The vapor and liquid densities (and therefore molar volumes) are not available. Predict the vapor pressure p in bar of liquid water at T = 20°C, 30°C, 80°C, 90°C. The approximate Clausius-Clapeyron equation can be used, but you should give the physical assumptions on which it is based. Hvap / RT 2 Answer: is based on (d ln p / dT ) (a) V vap V gas V liq V gas and (b) V g RT / P .furthermore, we will use the integrated form based on (c) H vap independent of T over a short range of T. Which we can compare with actual measured values below: T2 = 20°C = 293.15K –3 –3 –3 ln(p/6.11 x 10 ) = –5418.6 (3.411 x 10 – 3.661 x 10 ) = 1.353 –3 p = 3.871 (6.11 x 10 ) = 0.0237 bar at 20°C 0.0233 bar T2 = 30°C = 303.15K –3 –3 –3 ln(p/6.11 x 10 ) = –5418.6 (3.299 x 10 – 3.661 x 10 ) = 1.963 –3 p = 7.122 (6.11 x 10 ) = 0.0435 bar at 30°C 0.0424 bar T2 = 80°C = 353.15K Now use H vap at 100°C! –3 –3 ln p = –4890.5 (2.832 x 10 – 2.680 x 10 ) = –0.742 or p = 0.476 bar at 80°C 0.4733 bar T2 = 90°C = 363.15K –3 –3 ln p = –4890.5 (2.754 x 10 – 2.680 x 10 ) = –0.360 or p = 0.697 bar at 90°C 0.701 bar –1 Note: If one assumed H vap = a + bT, then a = 57,041.5 J and b = –43.90 J K . Integrating the approximate Clapeyron equation for this choice of H yields which is a better integrated form.