Ch 05 Thermodynamics mod 25
advertisement

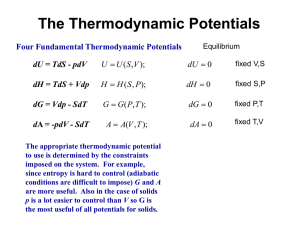
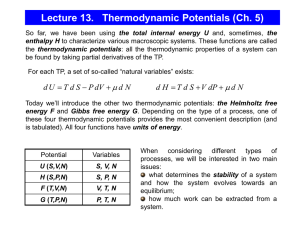
Thermodynamics A system: Some portion of the universe that you wish to study • The surroundings: The adjacent part of the universe outside the system Changes in a system are associated with the transfer of energy Natural systems tend toward states of minimum energy Basic Thermodynamics The theoretical basis of phase equilibrium Three Laws of Thermodynamics 1. First Law: Change in Energy dE = dQ – dW Q – heat energy W – work = F * distance (notice distance is a length) Pressure has units Force/Area = (F/dist2) so Work = F * dist = P * area * dist = P * V At constant pressure dW = PdV So if Pressure is constant: (1) dE = dQ – PdV where dV is thermal expansion or contraction Does not depend on path The symbol d means “the small change in” Energy E Kinetic + potential energy + Pressure energy + …. Kinetic energy due particles motion (vibration (heat), rotation, translation) Potential energy: position, rest mass energy, charge of atoms, energy of bonds. Changed by heating or by doing work on system Pressure energy = Pressure x unit Volume First law: dE = dQ – dW , change in energy is equal to the total heat added/removed and work done by/on the surroundings. If the system is isolated from its surroundings, its energy cannot change. Second and Third Laws of Thermodynamics 2. All substances move to the greatest state of disorder (highest Entropy ”S”) for a particular T and P. (2) dQ/T = dS “The state of greatest order [lowest S] is at the lowest temperature. With increasing temperature, disorder becomes more prevalent.” Minerals with simple atomic structure and simple chemistry have lower entropy. 3. At absolute zero (0ºK), Entropy is zero Does this suggest how to measure S? Can we measure changes in S by keeping track of temperature as we add heat to a system? “The entropy of a pure crystalline substance can therefore be obtained directly from heatcapacity measurements by assuming that S0 (at 0o K) is zero” Wood and Fraser (1978) p38 Stability of a phase (or mineral) is partly related to its internal energy (here “E”), which strives to be as low as possible under the external conditions. Metastability exists in a phase when its energy is higher than P-T conditions indicate it should be. (1) Activation Energy is the energy necessary to push a phase from its metastable state to its stable state. (2 minus 1) Equilibrium exists when the phase is at its lowest energy level for the current P-T conditions. (3) (Two minerals that are reactive with one another, may be found to be in equilibrium at particular P-T conditions which on phase diagrams are recognized as phase boundaries) Note: most metamorphic and igneous minerals at the earth’s surface are metastable. Example: Exsolution of K-spar and Albite at low temperatures; they were in Solid Solution at higher temperatures. Moles (mol) A number ~6.022 x 1023 of anything A mole of a substance is equal to its molecular mass in grams. Thus Aluminum has a molecular mass of ~26.98, so a mole of aluminum metal has a mass of 26.98 grams. Enthalpy H Enthalpy (symbol H) is the sum of internal energy plus work* related to changes in pressure and volume. H = E + PV The change in Enthalpy DH = Cp DT Cp is the Heat Capacity at constant pressure P. Cp the amount of heat energy required to raise 1 mole of a substance by 1oC. SI units Joules T is the Temperature in Kelvin *The energy required to "make room for it" by displacing its surroundings. Entropy S Entropy, S, is a measure of the disorder of a system. Example: A liquid (maybe a melt) is more disordered than a solid. SI units Joules/K Gibbs Free Energy Define G G = E + PV – TS The internal energy E might be thought of as the energy required to create a system in the absence of changes in temperature or volume. An additional amount of work PV must be done if the system is created from a very small volume in order to "create room" for the system. A system at constant temperature T will contain an amount of energy TS Gibbs is the energy in excess of that energy TS Stability and Gibbs Free Energy G G = E + PV – TS and we just defined enthalpy G(p,T) = H − TS H = E + PV so thermal energy minus Temp x disorder Stable forms of a chemical system have the minimum possible Gibbs Free Energy H is the Enthalpy, a measure of Thermal Energy S is the Entropy, a measure of randomness, of probability Changes Thermodynamics treats changes Regardless of path G = E + PV – TS We should rewrite the equation for Gibbs Free Energy in terms of changes, D G DG = E + PDV – TDS DG = DH for P, T constant – TDS D = pronounced “delta” means “the change in” The change in Gibbs free energy, ΔG, in a reaction is a very useful parameter. It can be thought of as the maximum amount of work obtainable from a reaction. DH can be measured in the laboratory with a calorimeter. DS can also be measured with heat capacity measurements. Values are tabulated in books. Gibbs for a chemical reaction Hess’s Law applied to Gibbs for a reaction 298.15K, 0.1 MPa DG = ni G ( products) ni G (reactants ) 0 R 0 i i 0 i i Suppose 3A + 2B = 2C +1D reactants = products DG = 2GC +1GD -3GA – 2GB Gibbs Free Energy (G) is measured in KJ/mol or Kcal/mol One small calorie cal ~ 4.2 Joules J Molar Gibbs Free Energy of Formation G extensive, depends on amount Make intensive by using molar free energy, Joules/mole Measure H with calorimetry and S (measure DQ/T from T = 0 to 298K and define S0K = 0). More precision at low T’s Conditions 298K and 0.1 MPa = 1 bar are called “the reference state”. For the reaction Si + O2 = SiO2 Gf = Hf – TSf = -856.3 kJ/mol 298K and 0.1 MPa Gibbs free energy of formation The Gibbs free energy of formation is Gf° = Hf° - TfS° For example, to calculate the Gibbs free energy of formation of anhydrite, CaSO4 , we can use Hf°CaSO4 = -1434.11 kJ/mole S°CaSO4 = 106.7 J mol-1 K-1 S°Ca = 41.42 J mol-1 K-1 Hfo Ca = 0 S°S = 31.80 J mol-1 K-1 Hfo S= small S°O2 = 205.138 J mol-1 K-1 Hfo O2= 0 and we calculate the entropy of formation of anhydrite Sf° = S°CaSO4 - S°Ca - S°S - 2 * S°O2 = -376.796 J mol-1 K-1 and then use Gf° = Hf° - TfSf° = -1434,110 - 298.15 * -376.796 = -1321.77 kJ/mol G for reactions DGR = DHR – TDSR Again: For a reaction A B That is, for reactants products When DGR is negative the forward reaction has excess energy and will occur spontaneously A B When DGR is positive there is not enough energy in the forward direction, and the back reaction will occur B A When DGR is zero reaction is at equilibrium, both reactions occur equally. Ex: Oxidation of Ferrous Iron, Fe+2 Groundwater: iron in two oxidation states Reduced ferrous iron (Fe+2) Oxidized ferric iron (Fe+3). Modern atmosphere 21% oxygen, so most O2 in shallow soils ferric state, (Fe+3). With time, mineralized. Fe+2 => Fe+3 Fe+3 is less soluble, forms oxides, hydroxides Initially Ferric Hydroxide (Fe(OH)3) amorphous hydrous ferric oxide (Fe2O3•xH2O), Hematite (Fe2O3), and Goethite FeO(OH). Gibbs Calc 1: Fe+2 => Fe+3 Oxidation of ferrous ion to ferric ion DG0R = DH0R – TDS0R DGR0 = ni Gi0 ( products) ni Gi0 (reactants ) i i H2O(l)=-63.32 kcal/mol = -63320 cal/mol You look these up in tables Reactants Products Fe2+ + ¼ O2 + H+ Fe3+ + ½ H2O =[-4120+(-63320*0.5)]-[-21870+(3954*0.25)] =[-67440]-[-20887.5]=-46557.5 cal/mol Negative, so forward (left to right) reaction will proceed Gibbs details dE = dQ – PdV dQ/T = dS, d(uv) = udv + vdu 1st Law so dQ = TdS 2nd Law G = E + PV – TS Differentiating dG = dE +PdV +VdP -TdS - SdT dE = dQ - PdV = TdS – PdV so dG = TdS – PdV + PdV + VdP -TdS – SdT dG = VdP –SdT All other terms cancel. Gibbs Calc 2: SiO2 at elevated P and T Suppose we want G for Quartz at 500oC and 500MPa Doesn’t depend on path, can do Pressure and Temperature separately dG = VdP –SdT we just saw. Or approximately DG = VDP –SDT Gp2 – Gp1 = V(P2 – P1) if T, V const T constant means DT = 0, V constant is a good approximation for solids Variation in Gibbs of a phase with T and P Doesn’t depend on path, do Pressure and Temperature separately PRESSURE calc, assume volume const for solids Gp2 – Gp1 = V(P2 – P1) if T, V const For P2 = 500 MPa, P1 = 0.1 MPa, V = 22.69 x 10-6 m3, Gp1 = - 856,300 J (lo-qtz looked up in a table) Gp2 = Gp1 + V(P2 – P1) = -856,300J + 22.69 x 10-6m3(500x106 – 0.1x106 = - 844,957 Joules ~ -845 KJ Data from Robie and Waldbaum (1970), not Table 5.1 in the text SiO2 at Elevated P and T Pa) Variations Gibbs of a phase with T and P Doesn’t depend on path, do Pressure and Temperature separately Assume S constant over change temperature error is small DG = VDP –SDT GT2 – GT1 = -S(T2 – T1) if P = 500 MPa const G500MPa, 773K = GT1 –S0.1MPa(773K – 298K) = -844957 J – 41.46 J/K (773 -298K) = -864650.5 Joules G SiO2 at 500MPa and 773K ~ -864.7 KJ P constant means DP = 0, S constant is a rough approximation provided DT not too big Gibbs Free Energy We defined G – the energy of a system in excess of its internal energy E. This is the energy necessary for a reaction to proceed. H = E + PV G = E + PV – TS = H – TS Now let’s derive some useful relationships. Differentiating dG = dE +PdV +VdP -TdS - SdT dE = dQ - PdV = TdS – PdV so dG = TdS – PdV + PdV + VdP -TdS – SdT = VdP –SdT This is equation dQ =TdS is the 2nd law (3) dG = VdP –SdT Reactions go to the phase with lower G If T = constant dT = 0, then dG = VdP (2) at constant T (dG/dP)T = V (dense (low V) phases are favored at high P) If P = constant dP = 0, then dG = -SdT (3) at constant P (dG/dT)P = -S (disordered phases (high S) are favored at high T) We saw (equation 3) that dG = VdP –SdT The Clapeyron Equation • defines the state of Equilibrium between reactants and products in terms of S and V From Eqn.3, if dG =0, dP/dT = ΔS / ΔV (eqn.4) The slope of the equilibrium curve on a P T diagram will be positive if S and V both decrease or increase with increased T and P The function G can be represented graphically on P T diagrams Constructing Phase Diagrams One Component: H2O Pressure-Temperature Phase Diagram The Gibbs and Clapeyron Equations allow us to estimate phase diagrams with extrapolations from laboratory measurements. The lines show where equilibrium conditions (DG = 0) occur. Clapeyron tells us the slope Dense (low V) phases are favored at high P Disordered phases (high S) are favored at high T dP/dT = ΔS / ΔV Clapeyron 1.Does the liquid or solid have the larger volume/unit mass? Usually liquid. (except H2O) Low V usually solid. 2. High pressure favors low volume, so which phase should be stable at high P? Solid 5. Both DV and DS increase to right. We can thus predict that the slope of solidliquid equilibrium should be positive and that increased pressure raises the melting point. 3.Does liquid or solid have a higher entropy? liquid 4. High temperature favors randomness, so which phase should be stable at higher T? Liquid is more random, expect at high T. Does the liquid or solid have the lowest G at point A? Solid, pt. A is in the solid field. What about at point B? Figure 5-2. Schematic P-T phase diagram of a melting reaction. Winter (2001) An Introduction to Igneous and Metamorphic Petrology. Prentice Hall. Liquid, same argument The phase assemblage with the lowest G under a specific set of conditions is the most stable The Payoff • Our experiments and calculations allow us to construct the 3-D plot in (a), and to project the mineral with the lowest free energy at each PT onto the graph in (b). Notice the points a,b and c at right. At c, DG = 0 in the reaction Andalucite Sillimanite Nesse fig 5.3 The KSA Phase diagram allows us to assign PT conditions to various Plate Tectonic settings Of Nesse