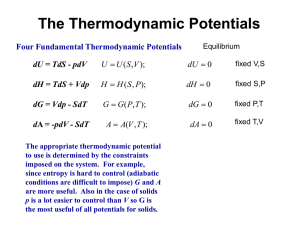
Basis concepts of Thermochemistry
advertisement
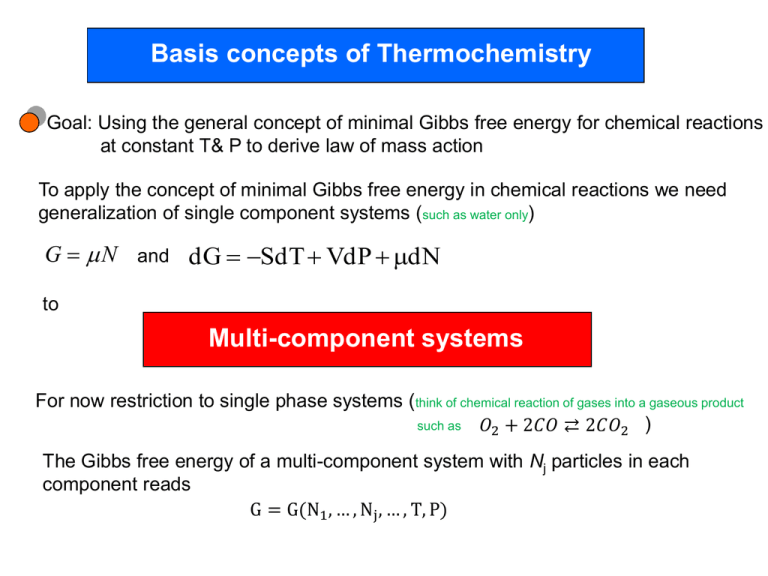
Basis concepts of Thermochemistry Goal: Using the general concept of minimal Gibbs free energy for chemical reactions at constant T& P to derive law of mass action To apply the concept of minimal Gibbs free energy in chemical reactions we need generalization of single component systems (such as water only) G N and dG SdT VdP dN to Multi-component systems For now restriction to single phase systems (think of chemical reaction of gases into a gaseous product such as 𝑂2 + 2𝐶𝑂 ⇄ 2𝐶𝑂2 ) The Gibbs free energy of a multi-component system with Nj particles in each component reads G = G(N1 , … , Nj , … , T, P) Using homogeneity of G according to 𝐺 𝜆𝑁1 , … , 𝜆𝑁𝑗 , … , 𝑇, 𝑃 = 𝜆𝐺 𝑁1 , … , 𝑁𝑗 , … , 𝑇, 𝑃 𝜕𝐺 𝜕𝐺 𝑁 +⋯ 𝑁 +⋯=𝐺 𝜕𝜆𝑁1 1 𝜕𝜆𝑁𝑗 𝑗 and especially for 𝜆 = 1 𝜕𝐺 𝜕𝐺 G = 𝜕𝑁 𝑁1 + ⋯ 𝜕𝑁 𝑁𝑗 + ⋯ = 1 𝑗 𝑗 𝜇𝑗 𝑁𝑗 as generalization of G N Likewise dG SdT VdP dN is generalized into dG = −SdT + VdP + 𝑗 𝜇𝑗 𝑑𝑁𝑗 On the way towards the law of mass action let’s derive a useful relation (Gibbs-Duham) between change of the chemical potentials as a result of particle exchange With dG = 𝑑 𝑗 𝜇𝑗 𝑁𝑗 = 𝑗 𝑑𝜇𝑗 𝑁𝑗 + 𝑗 𝜇𝑗 𝑑𝑁𝑗 dG = −SdT + VdP + and dG − dG = 0 = −SdT + VdP + 𝑗 𝜇𝑗 𝑑𝑁𝑗 - 𝑗 𝑑𝜇𝑗 𝑁𝑗 − 𝑗 𝜇𝑗 𝑑𝑁𝑗 𝑗 𝜇𝑗 𝑑𝑁𝑗 Gibbs-Duhem equation −SdT + VdP- When T and P are constant 𝑗 𝑗 𝑑𝜇𝑗 𝑁𝑗 𝑑𝜇𝑗 𝑁𝑗 = 0 =0 telling us that the chemical potentials of the different components are related For example: in a 2 component mixture when knowing 𝜇1 we can calculate 𝜇2 from 𝑁1 𝑑𝜇1 + N2 d𝜇2 = 0 Law of mass action Let’s consider a chemical reaction among species (components) For example the reaction 3𝐻2 + 𝑁2 ⇌ 2𝑁𝐻3 Dh=-92,5kJ/mol Let’s write this in the form -3𝐻2 − 𝑁2 + 2𝑁𝐻3 =0 Or in a general notation 𝜁1 𝐴1 + 𝜁2 𝐴2 + ⋯ + 𝜁𝜎 𝐴𝜎 = 0 stoichiometric coefficient For example: -3𝐻2 − 𝑁2 + 2𝑁𝐻3 =0 with Aj is a product if 𝜁𝑗 > 0 𝜁 𝑁𝐻3 = 2 and 𝑁𝐻3 product Aj is a reactant if 𝜁𝑗 < 0 𝜁 𝐻2 = −3 and 𝐻2 reactant , 𝜁 𝑁2 = −1 and 𝑁2 reactant Reaction goes in both directions: where is the equilibrium At constant T and P: equilibrium determined by G = G(N1 , … , Nj , … , T, P) minimum dG = 0 dG = −SdT + VdP + With 𝑗 𝜇𝑗 𝑑𝑁𝑗 =0 𝑗 𝜇𝑗 𝑑𝑁𝑗 and T,P constant in equilibrium If we look, e.g., at the forward direction of the reaction we identify 𝑗 𝜇𝑗 𝜁𝑗 𝑑𝑁𝑗 = 𝜁𝑗 =0 Consequences of this relation for ideal gases The chemical potential of an ideal gas can be derived from G implying ( 𝜕𝜇 1 𝜕𝐺 V k T )𝑇 = ( )𝑇 = = B 𝜕𝑃 N 𝜕𝑃 N P dG SdT VdP dN μ= N 𝜕𝜇 ( ) 𝑇 𝑑𝑃 + f(T) 𝜕𝑃 μ=𝑘𝐵 𝑇𝑙𝑛 𝑃/𝑃𝑟 + 𝑘𝐵 𝑇Φ(𝑇) For each component (reactant and product) of the chemical reaction we can write 𝑃𝑗 μ𝑗 =𝑘𝐵 𝑇𝑙𝑛 + 𝑘𝐵 𝑇Φj (𝑇) 𝑃𝑟 𝑗 𝜇𝑗 𝜁𝑗 With =0 (under the assumption that the components can be described by ideal gases) and partial pressure of component j 𝑁𝑗 𝑃𝑗 = 𝑃 = P [Aj ] 𝑁 concentration of component j total pressure 𝑃 = 𝑘𝐵 𝑇 𝑗 𝜁𝑗 (𝑙𝑛 𝑃 𝑗 𝜁𝑗 [ 𝑙𝑛 𝑗 𝑒 𝑃 𝑃𝑟 [𝐴𝑗 ] 𝑃𝑟 + 𝑙𝑛[𝐴𝑗 ] + Φj (𝑇)] =0 =𝑒 𝑃 𝜁𝑗 [ 𝑙𝑛 + Φj (𝑇) ] 𝑃 𝑗 𝑟 𝑃 − 𝑗 𝜁𝑗 [ 𝑙𝑛 𝑃 +Φj (𝑇) ] 𝑟 Law of mass action [𝐴𝑗 ]𝜁𝑗 = 𝜅(𝑃, 𝑇) 𝑗 according Dalton’s law + Φj (𝑇) ) =0 𝑙𝑛[𝐴𝑗 ]𝜁𝑗 = − 𝜁𝑗 𝑗 𝑙𝑛[𝐴𝑗 ] 𝑗 𝑃𝑗 Example: 3𝐻2 + 𝑁2 ⇌ 2𝑁𝐻3 𝑁𝐻3 2 =𝜅 𝐻2 3 𝑁2 where 𝜅(𝑃, 𝑇) Equilibrium constant Consequences from law of mass action in equilibrium [𝐴𝑗 ]𝜁𝑗 = 𝜅(𝑃, 𝑇) 𝑗 If 𝜅(𝑃, 𝑇) large requires 𝐴𝑗 large with 𝜁𝑗 > 0 meaning product concentration large 𝐴𝑗 small with 𝜁𝑗 < 0 meaning reactant concentration low If 𝜅(𝑃, 𝑇) small requires 𝐴𝑗 small with 𝜁𝑗 > 0 meaning product concentration low 𝐴𝑗 large with 𝜁𝑗 < 0 meaning reactant concentration large What can we say about P and T dependence of 𝜿 Can we use P to shift the equilibrium to the side of reactants or products 𝜅= 𝑒 𝑃 − 𝑗 𝜁𝑗 [ 𝑙𝑛 𝑃 +Φj (𝑇) ] 𝑟 𝑃 𝜅= 𝑃𝑟 If 𝑗 𝜁𝑗 − 𝑗 𝜁𝑗 = 𝑒 𝑃 −𝑙𝑛 𝑃 𝑟 𝑒 − 𝑗 𝜁𝑗 Φ𝑗(𝑇) = 0 no P dependence of 𝜅 𝑗 𝜁𝑗 − 𝑗 𝜁𝑗 Φj (𝑇) If 𝑗 𝜁𝑗 > 0 increase in P decreases 𝜅 Equilibrium shifts towards reactants If 𝑗 𝜁𝑗 < 0 increase in P increases 𝜅 Equilibrium shifts towards products Can we use T to shift the equilibrium to the side of reactants or products − 𝑗 𝜁𝑗 𝑃 𝜅= 𝑃𝑟 𝜕𝑙𝑛𝜅 𝜕𝑇 From μ𝑗 =𝑘𝐵 𝑇 𝑙𝑛 𝑇 𝑒− 𝑗 𝜁𝑗 =− 𝑃 𝑃 ln 𝜅 = 𝑙𝑛 𝑃𝑟 Φ𝑗 (𝑇) 𝑑Φ𝑗 𝜁𝑗 𝑑𝑇 𝑗 𝑃𝑗 + 𝑘𝐵 𝑇Φj (𝑇) 𝑃𝑟 𝜕μ𝑗 𝜕𝑇 𝑃 − 𝑗 𝜁𝑗 Φ𝑗 (𝑇) 𝑑Φ Next we replace by a quantity closely related to the 𝑑𝑇 experiment such as the heat released in an exothermal reaction 𝜕μ𝑗 𝜕𝑇 = μ𝑗 + 𝑘 𝐵 𝑇 2 − 𝑗 𝜁𝑗 𝑃 𝑃𝑗 𝑑Φ𝑗 = 𝑘𝐵 𝑙𝑛 + 𝑘𝐵 Φj 𝑇 + 𝑘𝐵 𝑇 𝑃𝑟 𝑑𝑇 𝑑Φ𝑗 𝑑𝑇 A From 𝐺 = 𝐻 + 𝑇𝑆 𝜕μ𝑗 𝜕𝑇 μ𝑗 = ℎ𝑗 + 𝑇𝑠𝑗 = 𝑠𝑗 𝑃 using 𝜕μ𝑗 μ𝑗 = ℎ𝑗 + 𝑇 𝜕𝑇 dG SdT VdP dN A B 𝑃 & B 𝑑Φ𝑗 hj = − 𝑘𝐵 𝑇 𝑑𝑇 2 From 𝜕𝑙𝑛𝜅 𝜕𝑇 𝑑Φ𝑗 𝜁𝑗 𝑑𝑇 𝑗 =− 𝑃 𝜕𝑙𝑛𝜅 𝜕𝑇 = 𝑃 𝜕𝑙𝑛𝜅 𝜕𝑇 and hj = − 𝑘𝐵 𝑇 2 𝑑Φ𝑗 𝑑𝑇 𝑗 𝜁𝑗 ℎ𝑗 𝑘𝐵 𝑇 2 𝑃 Δℎ = 𝑘𝐵 𝑇 2 where 𝑗 𝜁𝑗 ℎ𝑗 = Δℎ is the enthalpy of the reaction For a better understanding let’s integrate the equation 𝜅 𝑇2 ln = 𝜅 𝑇1 𝑇2 𝑇1 Δℎ 𝑑𝑇 𝑘𝐵 𝑇 2 𝜅(𝑇2 ) = 𝜅 −Δℎ 1 1 − 𝑇1 𝑒 𝑘𝐵 𝑇2 T1 If Δℎ < 0 heat leaves the system, the reaction is exothermic 𝜅 decreases with increasing T and the yield goes down (consider the reaction of hydrogen and oxygen into water. At high temperature (about 6000K ) there is virtually no yield for water and at even higher T water dissociates into hydrogen and oxygen.) If Δℎ > 0 the reaction is endotherm 𝜅 increases with increasing T and the yield goes up The Haber-Bosch process An example to utilize the full potential of the law of mass action Industrial route to ammonia (Nobel Prize in Chemistry 1918, Fritz Haber) Let’s recall: 3𝐻2 + 𝑁2 ⇌ 2𝑁𝐻3 Dh=-92,5kJ/mol http://en.wikipedia.org/wiki/File:Fritz_Haber.png Fritz Haber, 1868-1934 At room temperature the reaction is slow (note: our equilibrium considerations say nothing about kinetics ! ) Is it a good idea to increase T to increase reaction speed Equilibrium consideration from law of mass action limits this possibility Since Δℎ < 0 reaction is exothermic 𝜅 decreases with increasing T and the yield goes down Compromise: T not higher than needed for catalyst to work (400 C) http://en.wikipedia.org/wiki/Haber_process Can pressure be utilized to favor the forward direction Law of mass action provides the answer: Yes Let’s recall -3𝐻2 − 𝑁2 + 2𝑁𝐻3 =0 𝜁1 𝐴1 + 𝜁2 𝐴2 + ⋯ + 𝜁𝜎 𝐴𝜎 = 0 𝑃 𝜅= 𝑃𝑟 𝑗 − 𝑗 𝜁𝑗 𝑒− 𝑗 𝜁𝑗 Means here 𝜁 𝑁𝐻3 = 2 𝜁 𝐻2 = −3 𝜁 𝑁2 = −1 Φ𝑗 (𝑇) 𝜁𝑗 = −3 − 1 + 2 = −2 < 0 high-pressure reactor (1913) in the ammonia plant of the Badische Anilin und Soda Fabrik (BASF) 𝜅 increases with increasing P Expenses/technical reasons of high P equipment bring limitations to this approach Compromise: 6–18 MPa (59–178 atm) Finally: we have seen from the discussion of stability conditions A perturbation away from equilibrium creates a force driving the system back (Le Chatelier’s principle) removing NH3 will increase yield of production 𝑁𝐻3 2 =𝜅 𝐻2 3 𝑁2 (In the Haber-Bosch process ammonia is removed as a liquid)