infection
advertisement
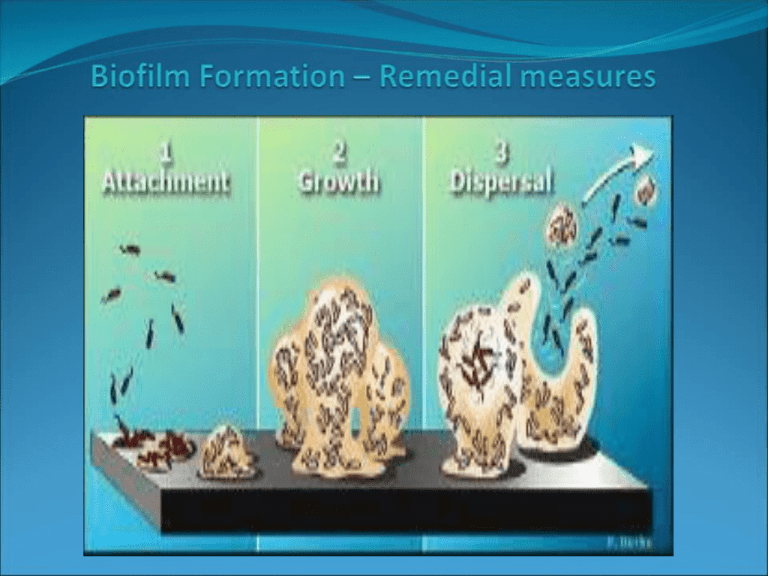
Introduction Biofilm • Assemblages of the microbial cells (Kolkare et al 2009) • Develop on all solid surfaces • Zobell (1943) • Adsorption of organic compounds • Enable diverse microbial communities (Marshall 1972) Problems • water processes, including corrosion, loss of process efficiency such as heat transfer, and health issues such as Legionella infection. Biofilm forming organism • bacteria, fungi, algae, protazoa Biofilms in the dairy industry • Dairy industry equipment can lead to serious hygiene problems and economic losses due to food spoilage and equipment impairment (Bremer, Fillery, & McQuillan, 2006; Gram, Bagge-Ravn, Ng, Gymoese, & Vogel, 2007). • Dairy biofilms are predominated by bacterial extracellular polymeric substances (EPS). Biofilms in humans • All surfaces of the body exposed to the external environment (skin, teeth, mouth, respiratory and gastrointestinal epithelia). • It has been estimated that 65% of human infections involve biofilms (Potera, 1999). • Staphylococcus epidermidis • Dental plaque is the biofilm that has received the most attention Bacterial Biofilm • Bacteria in nature are either in a planktonic state (“free-swimming”), or adhered to surfaces and become sessil (Joseph. L et al 2007). • Adopting sessile mode of Life o concentrate nutrients o metabolic interactions o resistance to harmful chemicals and environmental stress • P. aeruginosa Algal Biofilms • Biofilms dominated by algae will grow on any surface • In natural ecosystems, algal biofilms are familiar on trees and fences, on stones in rivers, and on the seashore. • unicellular, colonial or simple filamentous organisms • Diatoms are dominated in aquatic environment • Green unicellular algae is dominated in terrestrial environment. Fungal Biofilm • Transplantation procedures, immunosuppression, the use of chronic indwelling devices, and prolonged intensive care unit stays have increased the prevalence of fungal disease. • Candida is the major fungal pathogen of humans causing a variety of afflictions ranging from superficial mucosal diseases to deep seated mycoses. • Candida biofilms are difficult to eradicate especially because of their very high antifungal resistance Biofilm formation Biofilm formation comprises a sequence of steps (Breyers & Ratner, 2004). Processes governing biofilm formation (Breyers & Ratner, 2004). Courtesy from the American Society for Microbiology Properties • Specialized attachment structures/surface properties of the cell • Extracellular polymeric substances (EPS) • Cell–cell communication Approach for biofilm mitigation – biofilm prevention • preventing biofilm formation would be a more logical option than treating it. • no known technique. • The main strategy to prevent biofilm formation is to clean and disinfect regularly before bacteria attach firmly to surfaces (Midelet & Carpentier, 2004; Simo˜es et al., 2006). Cleaning and disinfection • • • • Disinfectants do not penetrate the biofilm matrix Therefore, cleaning is the first step. Surfactants or alkali products Disinfectants are more effective in the absence of organic material The green strategy for biofilms control – enzymes, phages and bioregulation Enzyme-based detergents • enzyme-based detergents as bio-cleaners, also known as ‘‘green chemicals’’ • Due to the EPSheterogeneity, a mixture of enzymes may be necessary for sufficient biofilm degradation • Lactobacillus bulgaricus, Lactobacillus lactis, Streptococcus thermophilus (Blum 2003) • proteases and polysaccharide hydrolysing enzymes may be useful (Meyer, 2003) Control using phages • Phages are ubiquitous in nature. • highly specific, non-toxic, feasible approach for controlling several microorganisms involved in biofilm formation (Kudva, Jelacic, Tarr, Youderian, & Hovde, 1999). • The technology for this has not yet been successfully developed. • A bacteriophage (L. monocytogenes phage ATCC 23074-B1) was used successfully in L. monocytogenes biofilm inactivation (Hibma, Jassim, & Griffiths, 1997). • This enzymatic phage had the ability to attack the bacterial cells in the biofilm and the biofilm matrix, substantially reducing the biofilm cell counts (more than 99.9% of removal). Control through microbial interactions/metabolite molecules • Metabolite can interfere with biofilm formation and development (Carpentier & Chassing, 2004; Kives et al., 2005; Røssland, Langsrud, Granum, & Sørhaug, 2005; Tait & Sutherland, 1998; Valle et al., 2006). • Many bacteria are capable of synthesizing and excreting biosurfactants with anti-adhesive properties (Desai &Banat, 1997; Nitschke & Costa, 2007; Rodrigues, van der Mei, Teixeira, & Oliveira, 2004; van Hamme, Singh, & Ward, 2006). • Surfactin from Bacillus subtilis disperses biofilms without affecting cell growth and prevents biofilm formation by microorganisms such as Salmonella enterica, E. coli, and Proteus mirabilis (Mireles, Toguchi, & Harshey, 2001). • Biopreservatives, such as nisin, lauricidin, reuterin and pediocin, have been well documented for their biofilm control potential against microorganisms commonly found in dairy processing facilities, including L. monocytogenes (Dufour et al., 2004; Garcia-Almendarez, Cann, Martin, Guerrero-Legarreta, & Regalado, 2008; Mahdavi, Jalali, & Kermanshahi, 2007; Zhao et al., 2004). • Valle et al. (2006) demonstrated that E. coli expressing group II capsules release a soluble polysaccharide into their environment that induces physicochemical surface alterations, which prevent biofilm formation by a wide range of Gram-positive and Gram-negative bacteria. • More recently, Davies and Marques (2009) found that P. aeruginosa produces cis-2- decenoic acid, which is capable of inducing the dispersion of established biofilms and of inhibiting biofilm development. Conclusions • Microbial control in food processing has the main aims of reduction/eradication of microbes and their activity, and the prevention/control of the formation of biological deposits on the process equipment. • Nowadays, the most efficient practical means for limiting microbial growth includes good production hygiene, a rational running of the process line, and effective use of cleaning and disinfectant products. • Due to the increased resistance of biofilms to conventional disinfection processes, novel means for their control are constantly sought through the control of environmental factors on the process line and the use of new control strategies. • Much more needs to be learned about the impact of antimicrobial products on microbial biofilms and their recovery responses to damage, as microorganisms can develop resistance and subsequently survive previously effective control procedures. • The discovery of new biofilm control strategies, following the specifications needed to be used in food industry, and based on the use of biological-based solutions with high antimicrobial activity and specificity seem to be a step ahead in overcoming the biofilm resistance issue. References • Aarnela, K., Lunde´ n, J., Korkeala, H., & Wirtanen, G. (2007). Susceptibility of Listeria monocytogenes strains to disinfectants and chlorinated alkaline cleaners at cold temperatures. LWT – Food Science and Technology, 40, 1041–1048. • Akiayama H, Huh W-K, Yamasaki O, Oono T, Iwatsuki K (2002) Confocal laser scanning microscopic observation of glycoclayx production by Staphylococcus aureus in mouse skin: does S. aureus generally produce a biofilm on damaged skin? Br J Dermatol 147: 879–85. • Bremer, P. J., Fillery, S., & McQuillan, A. J. (2006). Laboratory scale clean-in-place (CIP) studies on the effectiveness of different caustic and acid wash steps on the removal of dairy biofilms. International Journal of Food Microbiology, 106, 254–262. • Chae, M. S., Schraft, H., Truelstrup, L., & Mackereth, R. (2006). Effects of physicochemical surface characteristics of Listeria monocytogenes strains on attachment to glass. Food Microbiology, 23, 250–259. Chmielewski, R. A. N., & Frank, J. F. (2006). A predictve model for heat inactivation of Listeria monocytogenes biofilm on buna-N rubber. LWT – Food Science and Technology, 39, 11–19. • • Banin, E., Vasil, M. L., & Greenberg, P. (2005). Iron and Pseudomonas aeruginosa biofilm formation. Proceedings of the National Academy of Sciences USA, 102, 11076–11081. • Chae, M. S., Schraft, H., Truelstrup, L., & Mackereth, R. (2006). Effects of physicochemical surface characteristics of Listeria monocytogenes strains on attachment to glass. Food Microbiology, 23, 250–259. • Chaignon, P., Sadovskaya, I., Ragunah, Ch., Ramasubbu, N., Kaplan, J. B., & Jabbouri, S. (2007). Susceptibility of staphylococcal biofilms to enzymatic treatments depends on their chemical composition. Applied Microbiology and Biotechnology, 75, 125–132. • Dosti, B., Guzel-Seydim, Z., & Greene, A. K. (2005). Effectiveness of ozone, heat and chlorine for destroying common food spoilage bacteria in synthetic media and biofilms. International Journal of Dairy Technology, 58, 19–24. Dosti, B., Guzel-Seydim, Z., & Greene, A. K. (2005). Effectiveness of ozone, heat and chlorine for destroying common food spoilage bacteria in synthetic media and biofilms. International Journal of Dairy Technology, 58, 19–24. Drenkard, E., & Ausubel, F. M. (2002). Pseudomonas biofilm formation and antibiotic resistance are linked to phenotypic variation. Nature, 416, 740–743. • • Cont… • • • Dufour, M., Simmonds, R. S., & Bremer, P. J. (2004). Development of a laboratory scale clean-in-place system to test the effectiveness of ‘‘natural’’ antimicrobials against dairy biofilms. Journal of Food Protection, 67, 1438–1443. Dunstall, G., Rowe, M. T., Wisdom, G. B., & Kilpatrick, D. (2005). Effect of quorum sensing agents o the growth kinetics of Pseudomonas spp. of raw milk origin. Journal of Dairy Research, 72, 276–280. Dykes, G. A., Sampathkumar, B., & Korber, D. R. (2003). Planktonic or biofilm growth affects survival, hydrophobicity and protein expression patterns of a pathogenic Campylobacter jejuni strain. International Journal of Food Microbiology, 80, 1–10. • Lapidot, A., Romling, U., & Yaron, S. (2006). Biofilm formation and the survival of Salmonella typhimurium on parsley. International Journal of Food Microbiology, 109, 229–233. • Mahdavi, M., Jalali, M., & Kermanshahi, R. K. (2007). The effect of nisin on biofilm forming foodborne bacteria using microtiter plate method. Research in Pharmaceutical Sciences, 2, 113–118. • Meylheuc, T., Renault, M., & Bellon-Fontaine, M. N. (2006). Adsorption of a biosurfactant on surfaces to enhance the disinfection of surfaces contaminated with Listeria monocytogenes. International Journal of Food Microbiology, 109, 71–78. • Nitschke, M., & Costa, S. G. V. A. O. (2007). Biosurfactants in food industry. Trends in Food Science and Technology, 18, 252–259. • Rivardo, F., Turner, R. J., Allegrone, G., Ceri, H., & Martinotti, M. G. (2009). Antiadhesion activity of two biosurfactants produced by Bacillus spp. prevents biofilm formation of human bacterial pathogens. Applied Microbiology and Biotechnology, 83, 541–553. • Simo˜es, M., Bennett, R. N., & Rosa, E. A. S. (2009). Understanding antimicrobial activities of phytochemicals against multidrug resistant bacteria and biofilms. Natural Product Reports, 26, 746–757. • Valle, J., Re, D. S., Henry, N., Fontaine, T., Balestrino, D., Latour-Lambert, P., et al. (2006). Broad-spectrum biofilm inhibition by a secreted bacterial polysaccharide. Proceedings of the National Academy of Sciences USA, 103, 12558–12563. Thank You