EPIEN Medical Technical Bulletin. The Global Opportunity For
advertisement

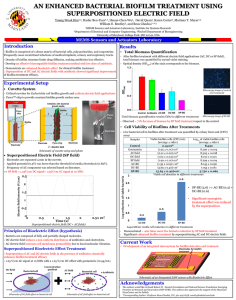
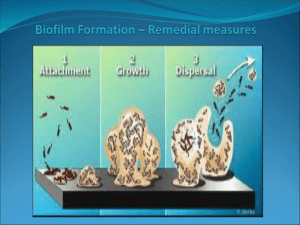
EPIEN Medical Technical Bulletin. The Global Opportunity For HYBENX® Technology to Reduce Pathogenic Biofilm J.W. Bracke, PhD and Michael L. Basara, MD Background Everything on Earth is covered with a living microscopic lawn of microorganisms called biofilm. Biofilm consists of a matrix of microbes interspersed with various biological molecules and debris that form a tenacious glue-like, living film on all surfaces. Biofilms are extremely resistant to disinfectants, detergents, and antimicrobial agents. The extent of biofilm was largely unappreciated until the 1990’s and the advent of recent microbial DNA analysis techniques. Now it is understood to be part of a dynamic genetic matrix that intimately interacts with the human genetic system as part of the human microbiome or “normal flora”. A “normal” healthy biofilm microbial flora is essential to good health. Fortunately, it is difficult to dislodge and eradicate from its host. The biofilm is composed of a balance of beneficial and potentially harmful microbes. The normal healthy balance can be disrupted by a decline in the overall health or hygiene of the host and lead to the development of an unhealthy pathogenic biofilm. Medical research has clearly demonstrated that this decline can lead to a variety of unhealthy medical conditions including pathogenic infections, ulcers, cardiovascular diseases, diabetes, prostatitis, obesity, surgical implant failures, etc. Death can result from the overgrowth of an unhealthy biofilm. Therefore, this complex phenomenon must be carefully managed during medical therapy. Antibiotic therapy, once thought to be the answer to managing these issues, is now contributing to worse outcomes due to overuse. We now know that antibiotic therapy can unbalance a healthy biofilm and cannot inactivate and remove all of the unhealthy biofilm components. As a result, surviving biofilm microbes (“persisters”) can acquire new resistance to each subsequent antibiotic usage that makes them even stronger and more deadly as they rebound from treatment. This phenomenon has led to an expensive spiral of new antibiotic drug development that is increasingly less effective against modern resistant infections. In addition, it is not focused and disrupts other non-target healthy biofilm elsewhere on the body. Conventional commercial antimicrobial development approaches have become a bankrupt strategy. A new simpler, less expensive, and more focused approach to pathogenic biofilm therapy is needed. EPIEN Medical’s novel HYBENX® Technology To address the increasing problem of resistant biofilms, EPIEN Medical has developed a novel, patented, safe, and effective topical chemical solution that is easily applied to any tissue surface to eradiate infectious biofilm in seconds. Because of the simple universal physical mechanism of the action of this product, development of biological resistance to this treatment is not possible. The topically applied product is a medical device. It is not a drug and has no residual or internal absorption effects. Because of this, biofilm outside of the treatment area is not affected. The scientific key to the beneficial action of EPIEN’s HYBENX® Solution comes from its carefully blended mixture of sulfonated component chemistry. These components cause the devitalization and denaturation of biofilm. The HYBENX sulfate components strongly absorb molecularly bound water. This is because water and sulfate have a unique complementary polarity and geometry of electrostatic charges on their respective surfaces. These surface charges strongly favor the mutual attraction of water and sulfates to form an intense thermodynamic hydrogen bond linkage. Water molecules form hydrogen bonds with many types of molecules, but the characteristics of this particular sulfate hydrogen bonding process are exceptional in strength, quality, and quantity. This makes the product’s desiccation power extremely fast, strong, and predictable. All of the bound water is instantly removed from vital organic biofilm components such as DNA, proteins, bacterial endotoxin, etc. Removal of water in this desiccation process results in instantaneous irreversible inactivation and denaturation of their biological function. The result is a coagulation of the entire biofilm matrix that destroys its attachment mechanisms to the underlying tissues and allows the biofilm to be easily removed by rinsing. Use of HYBENX® Solution HYBENX ® Solution (Liquid or Gel) is simple to use and accomplishes its cleansing action in seconds. It is applied to the biofilm surface for 5-20 seconds, at the clinician’s discretion, and removed by rinsing with aspiration. Depending upon the clinical situation, an application of HYBENX solution can further provide clinically significant secondary benefits. For example, biofilms can damage underlying tissues. After attacking the biofilm, the HYBENX Solution desiccating activity coagulates and seals underlying minor tissue lesions and reduces associated inflammatory edema. These actions often make patients more comfortable during and after medical treatment. Reduction of edema and swelling is often associated with reduced pain. The HYBENX desiccation mechanism has a minor transient effect on the underlying host tissue due to the natural resistance of intact protective human cell membranes to desiccation. A slight harmless surface blanching of the adjacent tissue may occur. This light surface desiccation will naturally reverse itself after a brief period, depending on the extent of the treated lesion. Product Development Activity ORAL BIOFILMS. One of the primary sources of all biofilm pathology is considered to be that found in the oral cavity. The development of an unhealthy oral biofilm primarily results from the lack of adequate oral hygiene (careful flossing and brushing). This can result in increasing pathology associated with tooth decay, periodontal disease, tooth loss, tooth pain, peri-implantitis, and ulceration. Current EPIEN research activity and a variety of published clinical reports (www.hybenx.it) have demonstrated that a single 5- to 20-second treatment of affected oral sites with HYBENX Solution is completely effective, as an adjunct agent with mechanical cleaning, in eradicating sub-gingival and supra-gingival biofilm, without the use of antibiotics. Clinicians evaluating the product routinely state that they have never seen another oral cleansing product demonstrate the instantaneous thorough cleaning and reversal of pathology and pain and suffering in their clinics that they experience with HYBENX products. They indicate that the product is easier, safer, and more effective to use than alternatives such as bleach, antibiotics, and laser therapies. In early controlled clinical studies, the pathology in periodontal disease has been significantly reduced when compared to conventional dental treatments. In further preliminary studies, HYBENX treatment also eliminated the pathogenic biofilm associated with periimplantitis around dental implants and alleviated pain and swelling within 7-10 days. In previous controlled research studies, HYBENX Technology also instantly eliminated the symptoms of aphthous ulcers (canker sores) with a single treatment and promoted rapid healing. Current Global Oral Product Status: 1) 2) 3) HYBENX has been granted EU and Canadian Clearance as a Class I Medical Device for general adjunctive oral cleanser applications throughout the oral cavity. An older product version of the HYBENX Technology, called Debacterol® Canker Sore Pain Relief, is accepted by FDA as a preamendment “drug” category for the treatment of aphthous ulcers EPIEN has received FDA 510(k) clearance for HYBENX® Oral Decontaminant as a Root Cleanser for use in endodontic therapy. The Company is in the process of trying to obtain a broader FDA clearance as a general cleanser throughout the mouth. Over seven million units of the HYBENX® Technology have been used safely and effectively. EPIEN is in the process of evaluating the prioritization of further research in several areas. These include: Infection Control. Extensive published medical studies have now shown that many internal medical problems are associated with the pathogenic microbes originating in oral biofilm. The Company is currently pursuing broader research opportunities to evaluate the technology’s ability to reduce the incidence of disseminated biofilm infections. This could be accomplished by using simple oral biofilm control procedures employing HYBENX Solution as a general oral biofilm control agent. Wound Biofilms. The association of pathogenic biofilms with the surface of chronic wounds is well established. This area is a major component of Infection Control efforts in medicine. Current healthcare practices and available products are not effective in controlling and resolving these chronic wounds. This has resulted in long-term care patients with expensive treatment histories. A chronic wound treatment specialist, Director of the CHRISTUS Hospital Centers Chronic Wound Treatment Clinics in Texas, has begun using HYBENX as a chronic wound debridement rinse in a variety of wound types. The treated wounds included pressure ulcers, diabetic ulcers, trauma, and nonhealing surgical wounds. Ten-second treatments with HYBENX were used to reverse patient’s months- or years-long histories of chronic wounds. The treated wounds reverted to a normal wound-healing timeline after one treatment. Two of the patients were removed from scheduled amputation surgery. This clinician, reminiscent of the oral clinicians experiences, stated that he has never before seen such a simple, easy-to-use, fast-acting, effective, pain-relieving product. These preliminary results have resulted in the signing of collaboration agreement with a major military research center. The Company is currently pursing an IND application with FDA to further investigate HYBENX as a wound debridement rinse. Skin Disorders. Extensive anecdotal reports have been supplied to the Company regarding the use of HYBENX Solution for the effective treatment of skin disorders such as acne, as well as dermatophyte infections (e.g., athlete’s foot) and other skin disorders and insect bites. The Company has completed initial studies in pig models that demonstrate the eradication of challenging wound infections such as MRSA, Pseudomonas, etc. Veterinary Applications. For all of the same reasons listed above there is strong interest by members of the veterinary clinical community in applications for HYBENX solutions. The Company is currently in discussions with veterinary distributors about the expansion of appropriate animal medical applications in the USA. Numerous veterinarians have already supplied the Company with results from several challenging animal applications where they have again received unique outcomes unprecedented with their previous veterinary products experiences. Current Product Status: Under US regulatory law, Veterinarians may currently purchase the human product for use in animals where the clinician believes there is no other current treatment available. Rev. 12Oct15